Mettiamoci daccordo su come interpretare le prove di



















- Slides: 60

Mettiamoci d’accordo su come interpretare le prove di funzionalità respiratoria Vito Brusasco Dipartimento di Medicina Interna e Specialità Mediche Scuola di Scienze Mediche e Farmaceutiche Università di Genova

ATS-ERS Task force on lung function testing Chair: Brusasco, Crapo, Viegi. 2001 -2005 1. General considerations for lung function testing Miller et al, ERJ Jul 2005 Cit. 747 2. Standardization of spirometry Miller et al. ERJ Aug 2005 Cit. 5437 3. Standardization of measurement of lung volumes Wanger et al. ERJ Sept 2005 Cit. 997 4. Standardization of the single-breath determination of carbon monoxide uptake in the lung Mac. Intyre et al. ERJ Oct 2005 Cit. 921 5. Interpretative strategies for lung function tests Pellegrino et al. ERJ Nov 2005 2270 Cit.

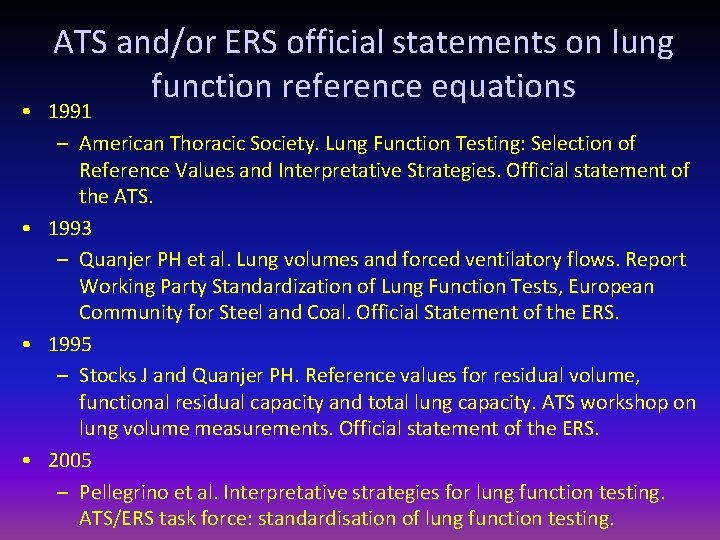
ATS and/or ERS official statements on lung function reference equations • 1991 – American Thoracic Society. Lung Function Testing: Selection of Reference Values and Interpretative Strategies. Official statement of the ATS. • 1993 – Quanjer PH et al. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the ERS. • 1995 – Stocks J and Quanjer PH. Reference values for residual volume, functional residual capacity and total lung capacity. ATS workshop on lung volume measurements. Official statement of the ERS. • 2005 – Pellegrino et al. Interpretative strategies for lung function testing. ATS/ERS task force: standardisation of lung function testing.


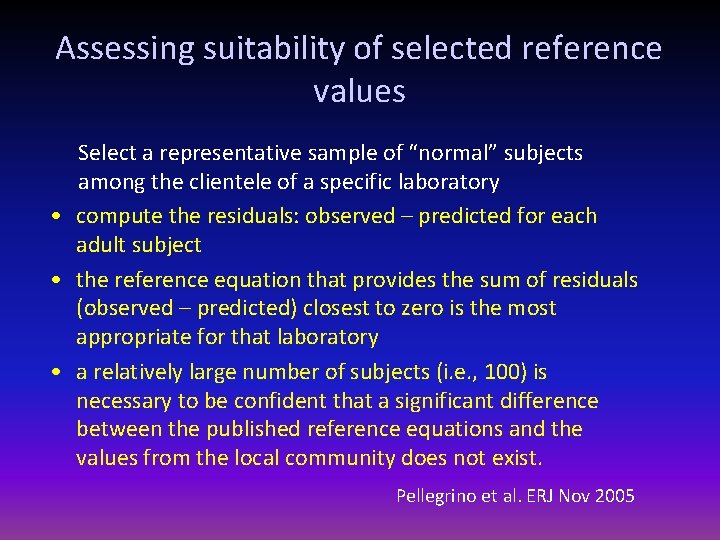
Assessing suitability of selected reference values Select a representative sample of “normal” subjects among the clientele of a specific laboratory • compute the residuals: observed – predicted for each adult subject • the reference equation that provides the sum of residuals (observed – predicted) closest to zero is the most appropriate for that laboratory • a relatively large number of subjects (i. e. , 100) is necessary to be confident that a significant difference between the published reference equations and the values from the local community does not exist. Pellegrino et al. ERJ Nov 2005

Effects of sample size and secular trends on derivation of reference equations Quanjer et al. ERJ 2011

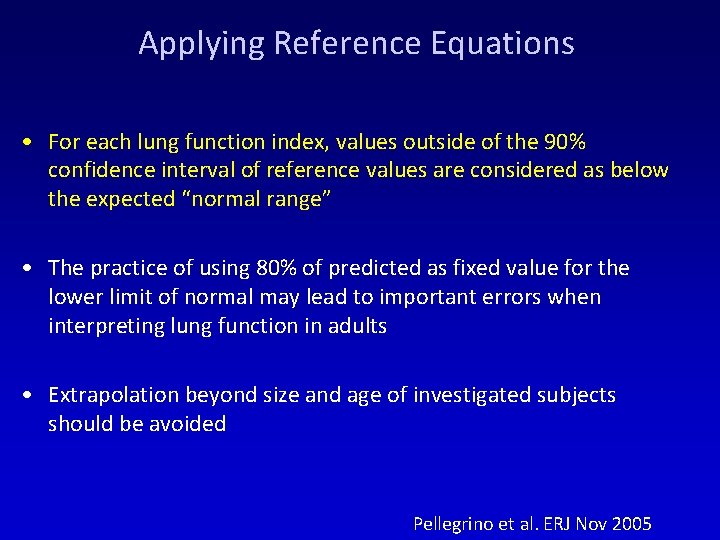
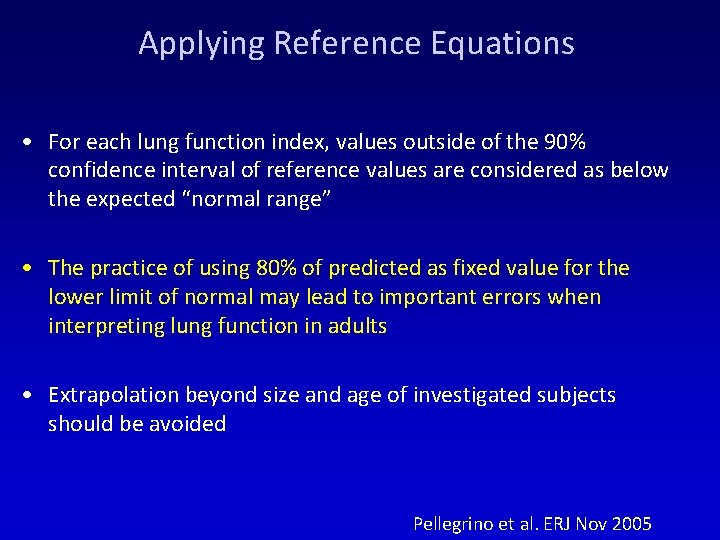
Applying Reference Equations • For each lung function index, values outside of the 90% confidence interval of reference values are considered as below the expected “normal range” • The practice of using 80% of predicted as fixed value for the lower limit of normal may lead to important errors when interpreting lung function in adults • Extrapolation beyond size and age of investigated subjects should be avoided Pellegrino et al. ERJ Nov 2005

The 90% confidence interval Normal Distribution Curve 1. 0 Mean = 0. 0 SD = 1. 0 Frequency 0. 8 0. 6 0. 4 0. 2 5% of population 0. 0 -5 -4 -3 -2 -1 0 1 Value of X 2 3 4 5

The limits of normality based on the 90% confidence interval Ref. Range = predicted ± 1. 645 x RSD includes 90% of normal observations (90% confidence limits) • 5% of normal population below lower limit of normal (LLN) (Mean -1. 645 x RSD) • 5% of normal population above upper limit of normal (ULN) (Mean +1. 645 x RSD) Thus, LLN will give 5% ‘false positives’ in a normal population

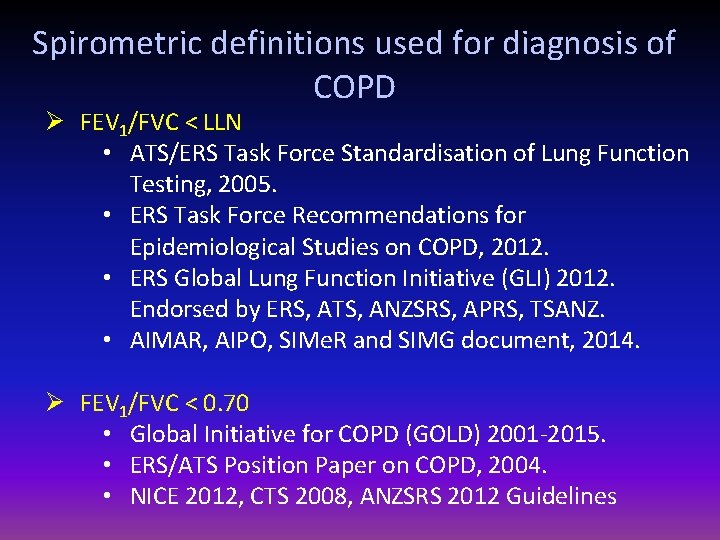
Spirometric definitions used for diagnosis of COPD Ø FEV 1/FVC < LLN • ATS/ERS Task Force Standardisation of Lung Function Testing, 2005. • ERS Task Force Recommendations for Epidemiological Studies on COPD, 2012. • ERS Global Lung Function Initiative (GLI) 2012. Endorsed by ERS, ATS, ANZSRS, APRS, TSANZ. • AIMAR, AIPO, SIMe. R and SIMG document, 2014. Ø FEV 1/FVC < 0. 70 • Global Initiative for COPD (GOLD) 2001 -2015. • ERS/ATS Position Paper on COPD, 2004. • NICE 2012, CTS 2008, ANZSRS 2012 Guidelines

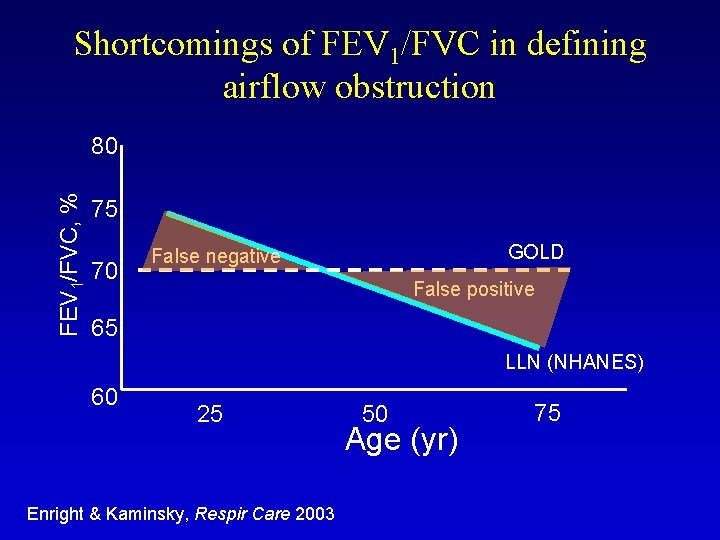
Shortcomings of FEV 1/FVC in defining airflow obstruction FEV 1/FVC, % 80 75 70 GOLD False negative False positive 65 LLN (NHANES) 60 25 Enright & Kaminsky, Respir Care 2003 50 Age (yr) 75

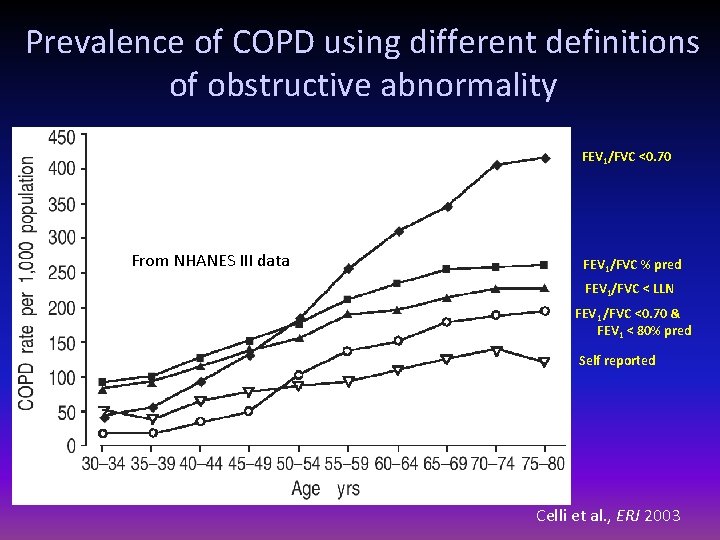
Prevalence of COPD using different definitions of obstructive abnormality FEV 1/FVC <0. 70 From NHANES III data FEV 1/FVC % pred FEV 1/FVC < LLN FEV 1 /FVC <0. 70 & FEV 1 < 80% pred Self reported Celli et al. , ERJ 2003

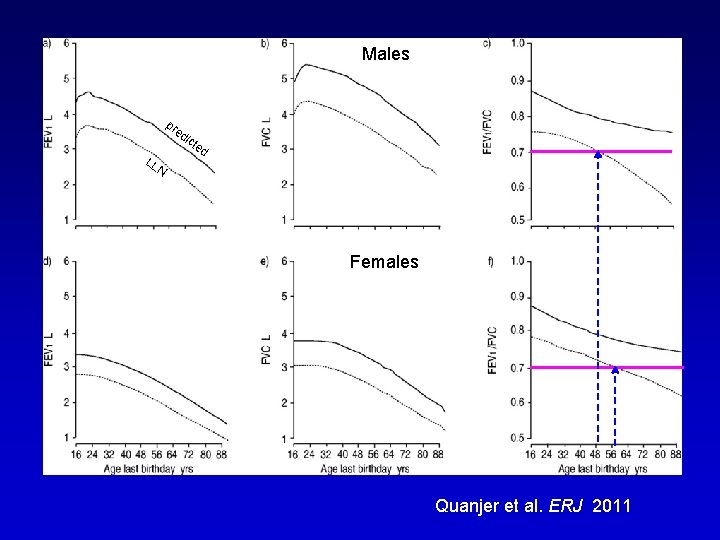
Males Maschi pr ed LL ict ed N Females Femmine Quanjer et al. ERJ 2011

Percentage of subjects with FEV 1/FVC<0. 70 Global Lung Initiative (n=78433) Quanjer et al, ERJ 2011

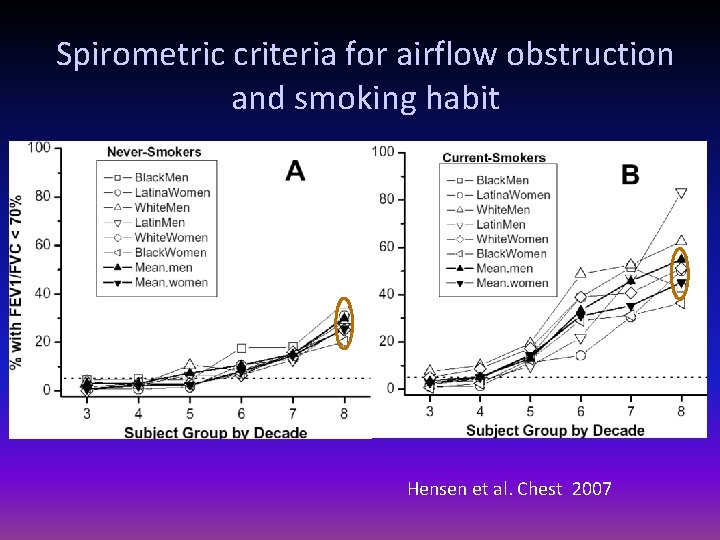
Spirometric criteria for airflow obstruction and smoking habit Hensen et al. Chest 2007

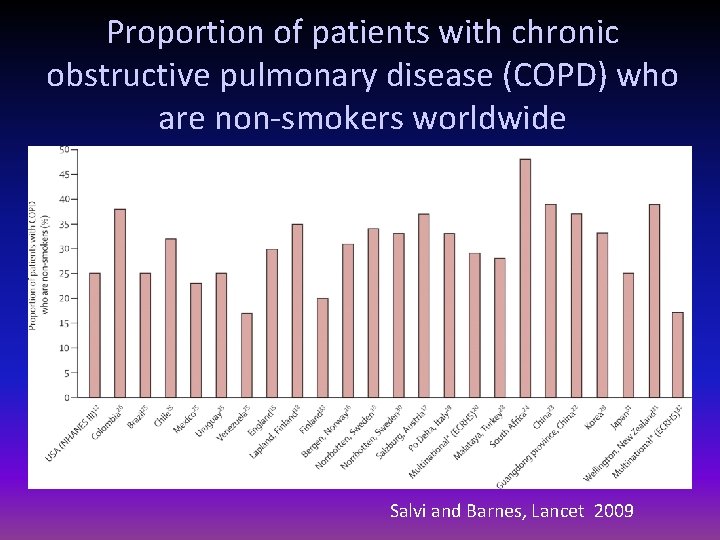
Proportion of patients with chronic obstructive pulmonary disease (COPD) who are non-smokers worldwide Salvi and Barnes, Lancet 2009

On the Road to Damascus? Pulm Med. 2011

GOLD 2011: Diagnosis of COPD “The spirometric criterion for airflow limitation remains a post-bronchodilator fixed ratio of FEV 1/FVC < 0. 70. It is recognized that the use of the fixed ratio (FEV 1/FVC) may lead to more frequent diagnoses of COPD in older adults with mild COPD as the normal process of aging affects lung volumes and flows, and may lead to underdiagnosis in adults younger than 45 years. ” Vestbo et al. AJRCCM 2012

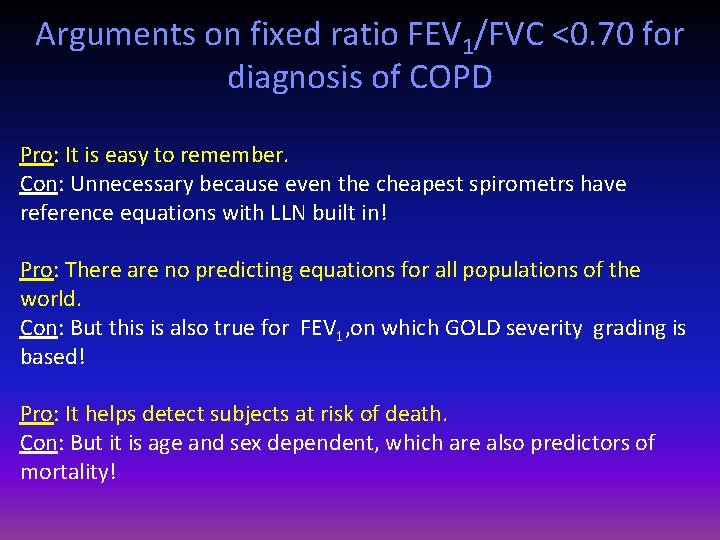
Arguments on fixed ratio FEV 1/FVC <0. 70 for diagnosis of COPD Pro: It is easy to remember. Con: Unnecessary because even the cheapest spirometrs have reference equations with LLN built in! Pro: There are no predicting equations for all populations of the world. Con: But this is also true for FEV 1, on which GOLD severity grading is based! Pro: It helps detect subjects at risk of death. Con: But it is age and sex dependent, which are also predictors of mortality!

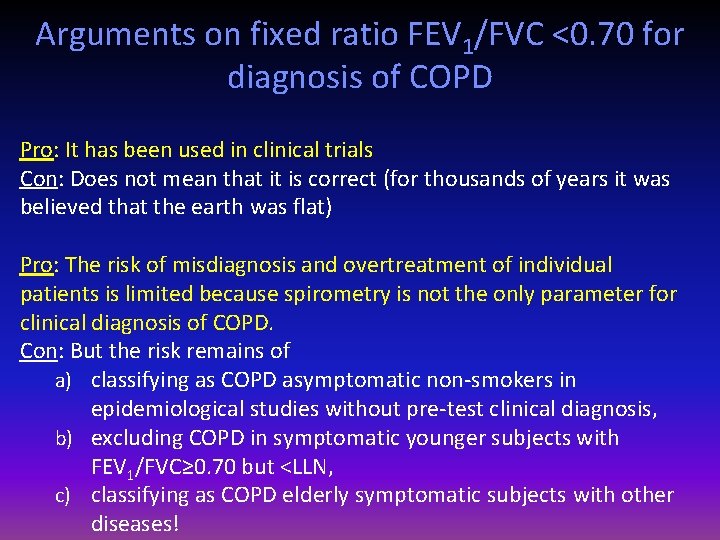
Arguments on fixed ratio FEV 1/FVC <0. 70 for diagnosis of COPD Pro: It has been used in clinical trials Con: Does not mean that it is correct (for thousands of years it was believed that the earth was flat) Pro: The risk of misdiagnosis and overtreatment of individual patients is limited because spirometry is not the only parameter for clinical diagnosis of COPD. Con: But the risk remains of a) classifying as COPD asymptomatic non-smokers in epidemiological studies without pre-test clinical diagnosis, b) excluding COPD in symptomatic younger subjects with FEV 1/FVC≥ 0. 70 but <LLN, c) classifying as COPD elderly symptomatic subjects with other diseases!

Suggested ways to reduce spurious prevalence of COPD using GOLD criteria 1. Only testing people with a high pre-test probability of COPD (clinical diagnosis, smoking history) 2. Confirming that airway obstruction persists after inhaling a bronchodilator (post-BD FEV 1) 3. Requiring that the FEV 1 be <80% of predicted (i. e. , GOLD II or more)

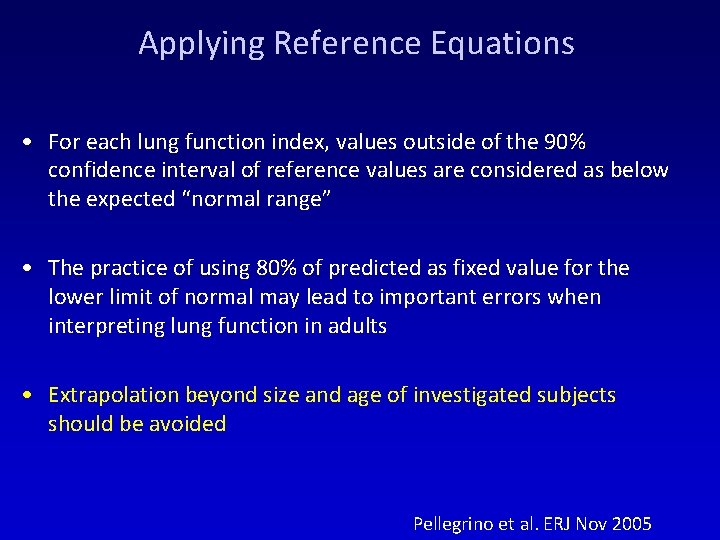
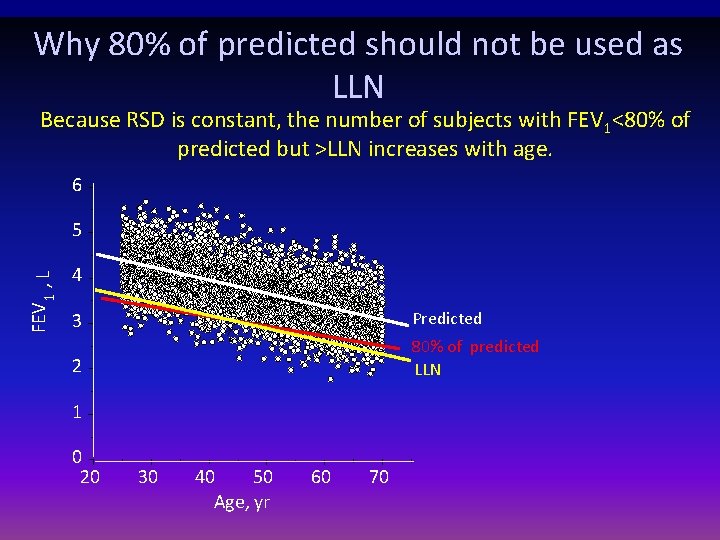
Applying Reference Equations • For each lung function index, values outside of the 90% confidence interval of reference values are considered as below the expected “normal range” • The practice of using 80% of predicted as fixed value for the lower limit of normal may lead to important errors when interpreting lung function in adults • Extrapolation beyond size and age of investigated subjects should be avoided Pellegrino et al. ERJ Nov 2005

Why 80% of predicted should not be used as LLN Because RSD is constant, the number of subjects with FEV 1<80% of predicted but >LLN increases with age. 6 FEV 1 , L 5 4 3 Predicted 2 80% of predicted LLN 1 0 20 30 40 50 Age, yr 60 70

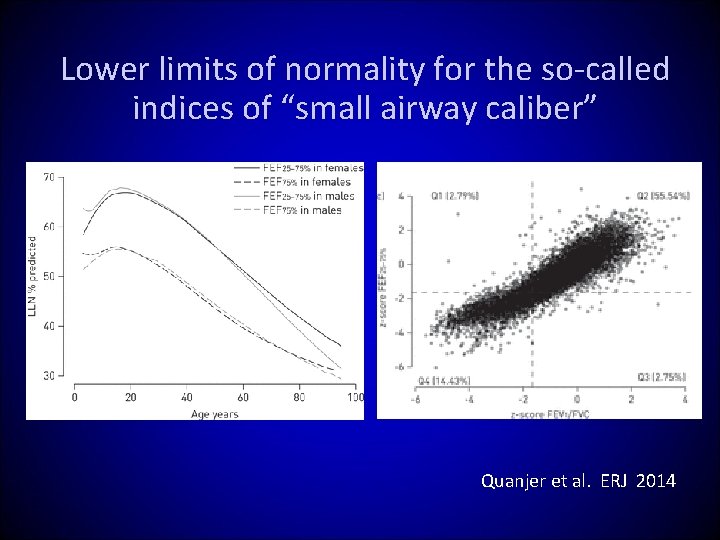
Lower limits of normality for the so-called indices of “small airway caliber” Quanjer et al. ERJ 2014

Age bias in the definition of airflow obstruction Healthy non-smoking East Asian females Quanjer et al. ERJ 2015

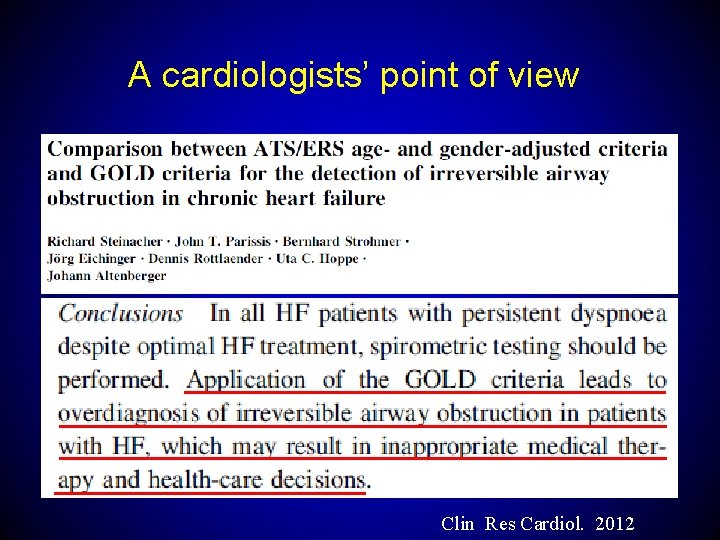
A cardiologists’ point of view Clin Res Cardiol. 2012

GOLD 2011 combined assessment of COPD

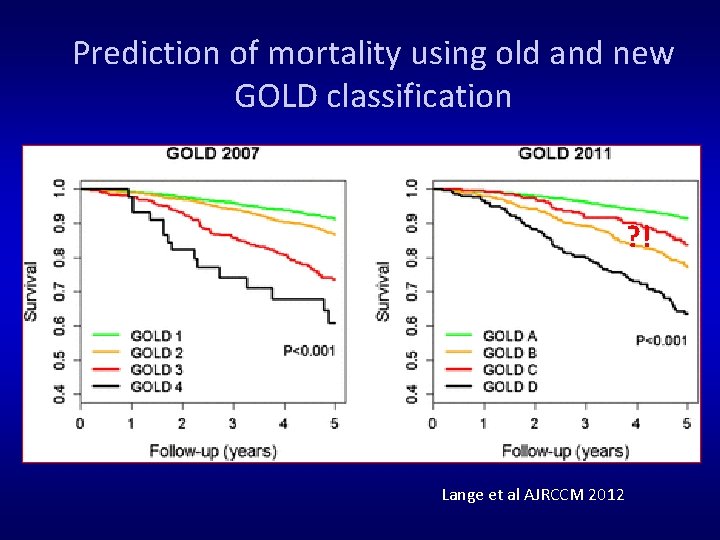
Prediction of mortality using old and new GOLD classification ? ! Lange et al AJRCCM 2012

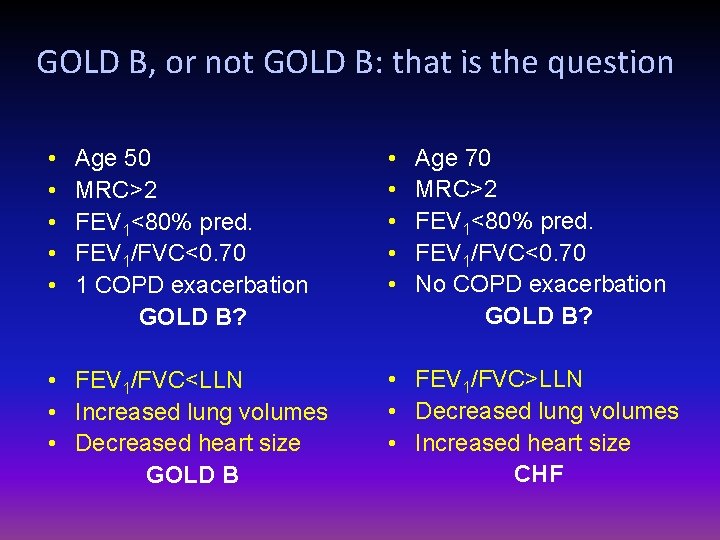
GOLD B, or not GOLD B: that is the question • • • Age 50 MRC>2 FEV 1<80% pred. FEV 1/FVC<0. 70 1 COPD exacerbation GOLD B? • FEV 1/FVC<LLN • Increased lung volumes • Decreased heart size GOLD B • • • Age 70 MRC>2 FEV 1<80% pred. FEV 1/FVC<0. 70 No COPD exacerbation GOLD B? • FEV 1/FVC>LLN • Decreased lung volumes • Increased heart size CHF

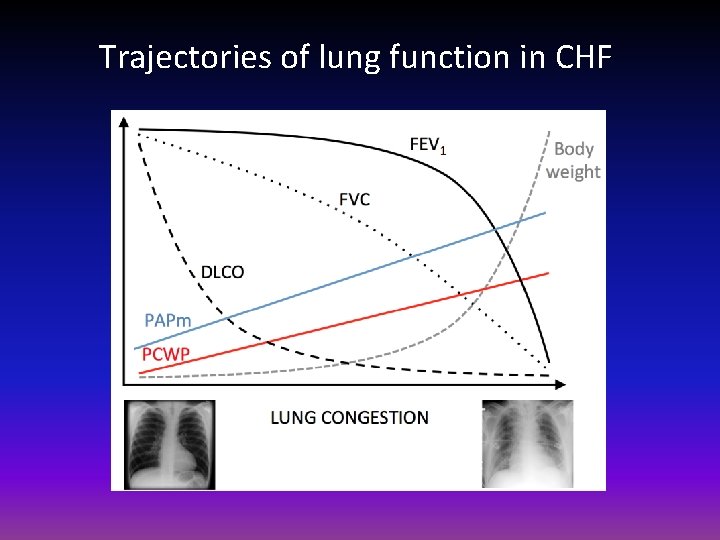
Trajectories of lung function in CHF

Applying Reference Equations • For each lung function index, values outside of the 90% confidence interval of reference values are considered as below the expected “normal range” • The practice of using 80% of predicted as fixed value for the lower limit of normal may lead to important errors when interpreting lung function in adults • Extrapolation beyond size and age of investigated subjects should be avoided Pellegrino et al. ERJ Nov 2005

Why 80% of predicted should not be used as LLN Because RSD is constant, the number of subjects with FEV 1<80% of predicted but >LLN increases with age. 6 FEV 1 , L 5 4 3 Predicted 2 80% of predicted LLN 1 0 20 30 40 50 Age, yr 60 70

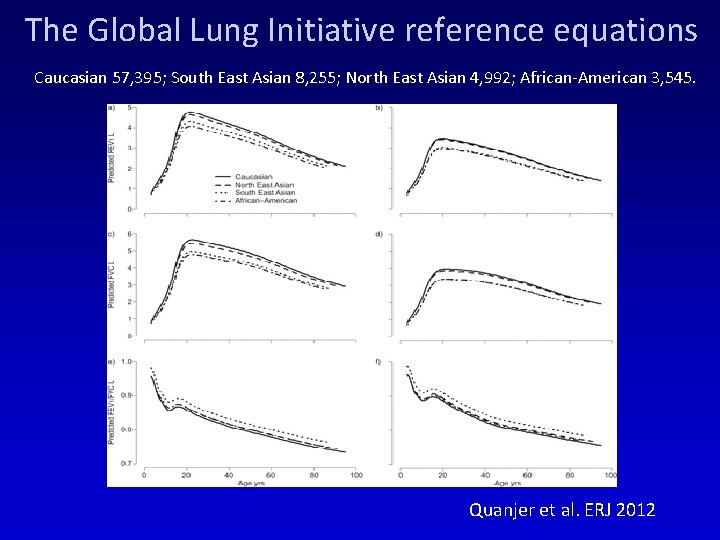
The Global Lung Initiative reference equations Caucasian 57, 395; South East Asian 8, 255; North East Asian 4, 992; African-American 3, 545. Quanjer et al. ERJ 2012

Additional Investigations Chest X-ray: Seldom diagnostic but valuable to exclude alternative diagnoses and establish presence of significant comorbidities. Lung Volumes and Diffusing Capacity: Help to characterize severity, but not essential to patient management. Oximetry and Arterial Blood Gases: Pulse oximetry can be used to evaluate a patient’s oxygen saturation and need for supplemental oxygen therapy. Alpha-1 Antitrypsin Deficiency Screening: Perform when COPD develops in patients of Caucasian descent under 45 years or with a strong family history of COPD. © 2015 Global Initiative for Chronic Obstructive Lung Disease

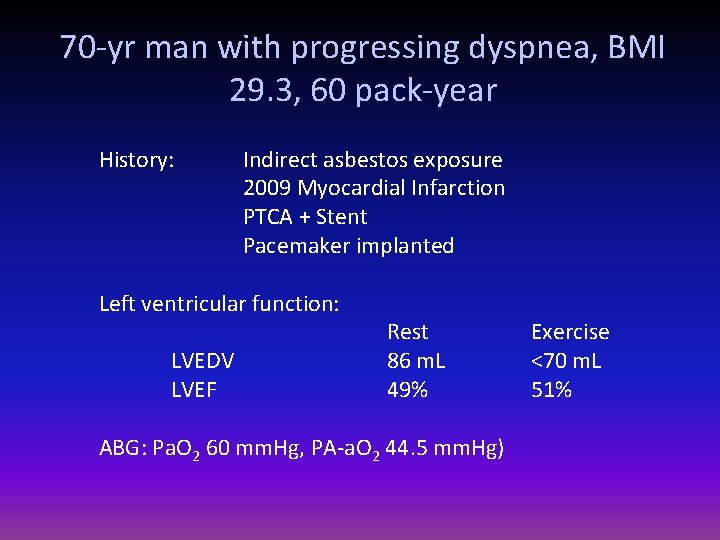
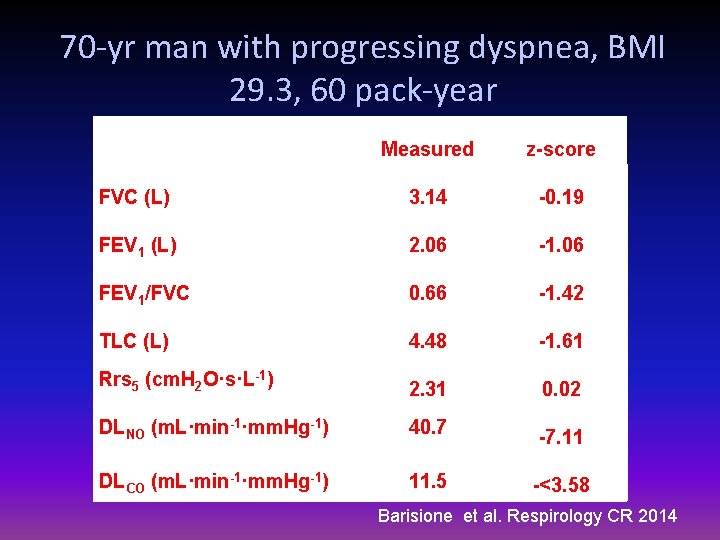
70 -yr man with progressing dyspnea, BMI 29. 3, 60 pack-year History: Indirect asbestos exposure 2009 Myocardial Infarction PTCA + Stent Pacemaker implanted Left ventricular function: LVEDV LVEF Rest 86 m. L 49% ABG: Pa. O 2 60 mm. Hg, PA-a. O 2 44. 5 mm. Hg) Exercise <70 m. L 51%

70 -yr man with progressing dyspnea, BMI 29. 3, 60 pack-year Measured z-score FVC (L) 3. 14 -0. 19 FEV 1 (L) 2. 06 -1. 06 FEV 1/FVC 0. 66 -1. 42 TLC (L) 4. 48 -1. 61 Rrs 5 (cm. H 2 O·s·L-1) 2. 31 0. 02 DLNO (m. L·min-1·mm. Hg-1) 40. 7 -7. 11 DLCO (m. L·min-1·mm. Hg-1) 11. 5 -<3. 58 Barisione et al. Respirology CR 2014

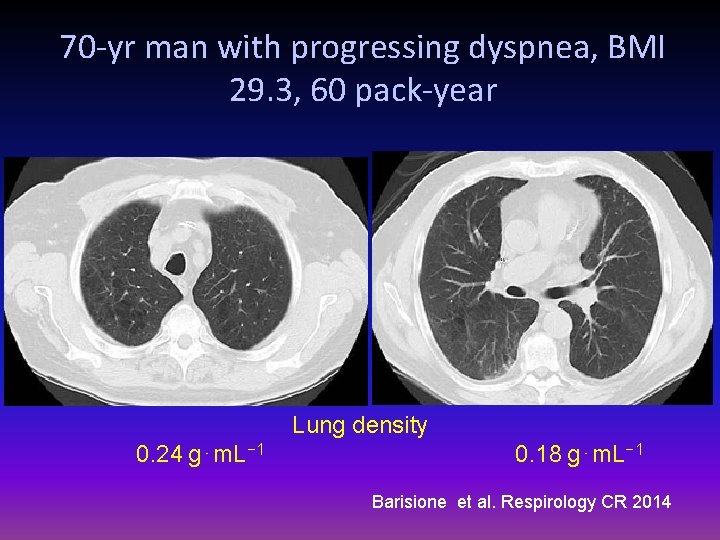
70 -yr man with progressing dyspnea, BMI 29. 3, 60 pack-year Lung density 0. 24 g⋅m. L− 1 0. 18 g⋅m. L− 1 Barisione et al. Respirology CR 2014

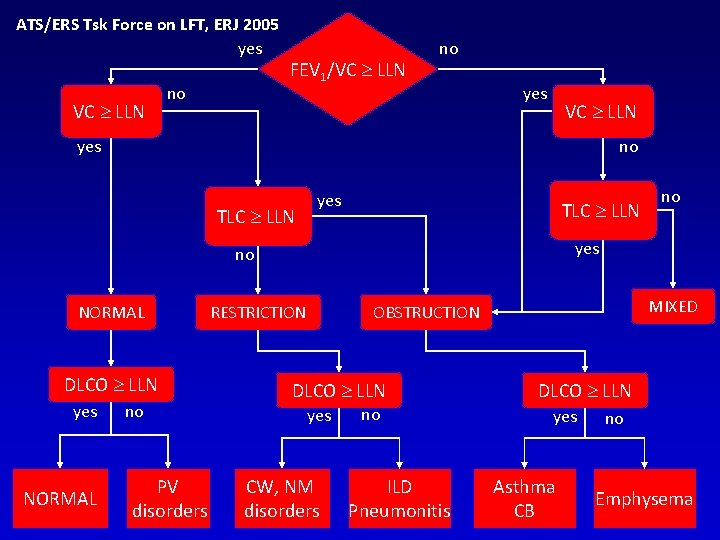
ATS/ERS Tsk Force on LFT, ERJ 2005 yes VC LLN no FEV 1/VC LLN no yes VC LLN yes no TLC LLN yes TLC LLN yes no NORMAL DLCO LLN yes NORMAL no PV disorders no RESTRICTION MIXED OBSTRUCTION DLCO LLN yes CW, NM disorders no ILD Pneumonitis DLCO LLN yes Asthma CB no Emphysema

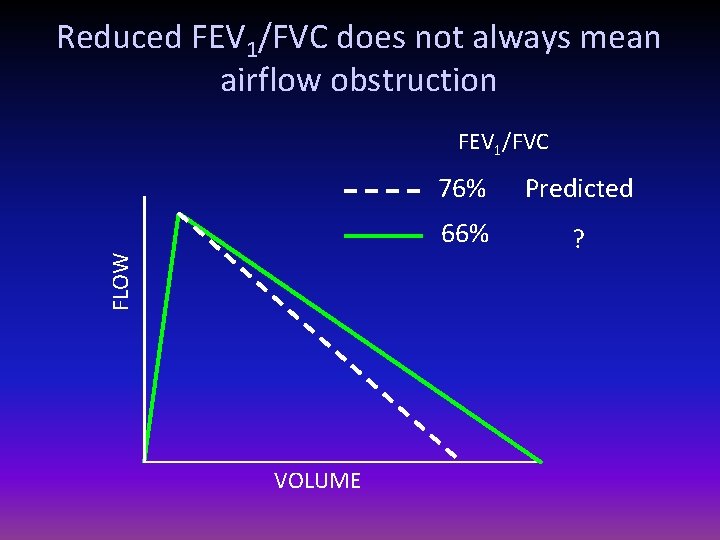
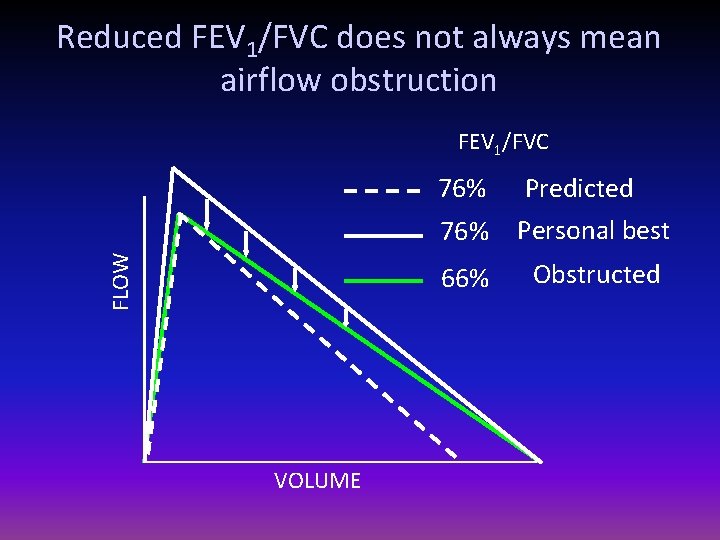
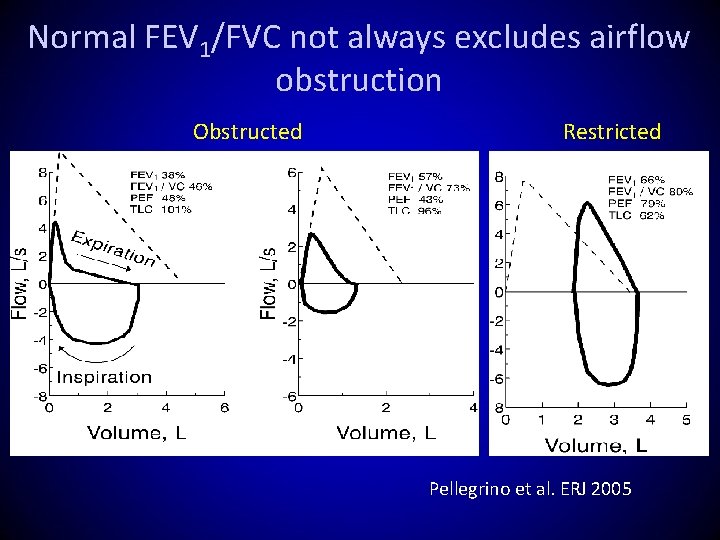
Reduced FEV 1/FVC does not always mean airflow obstruction FLOW FEV 1/FVC VOLUME 76% Predicted 66% ?

Reduced FEV 1/FVC does not always mean airflow obstruction FEV 1/FVC FLOW 76% VOLUME 76% Predicted Personal best 66% Obstructed

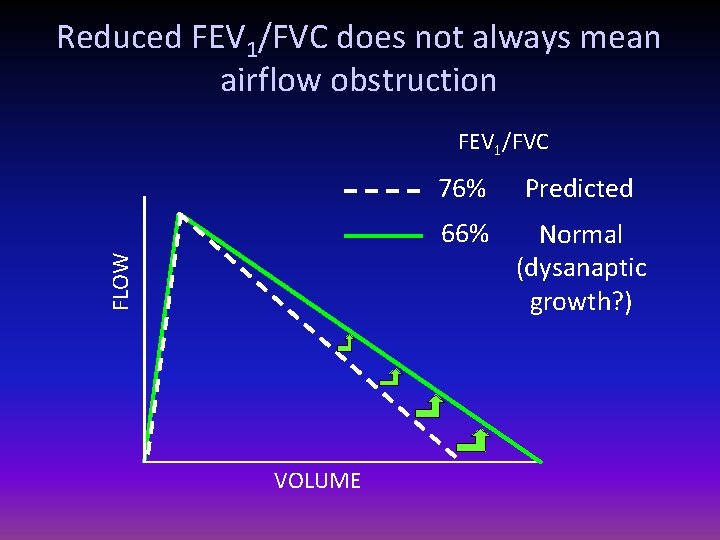
Reduced FEV 1/FVC does not always mean airflow obstruction FLOW FEV 1/FVC VOLUME 76% Predicted 66% Normal (dysanaptic growth? )

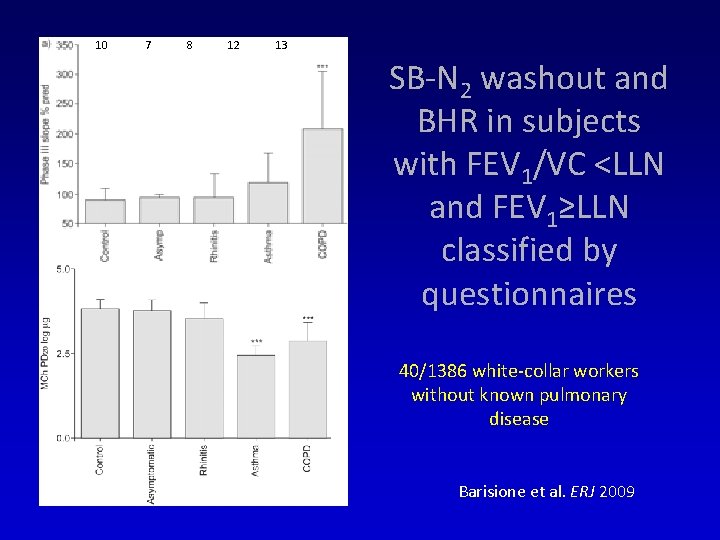
10 7 8 12 13 SB-N 2 washout and BHR in subjects with FEV 1/VC <LLN and FEV 1≥LLN classified by questionnaires 40/1386 white-collar workers without known pulmonary disease Barisione et al. ERJ 2009

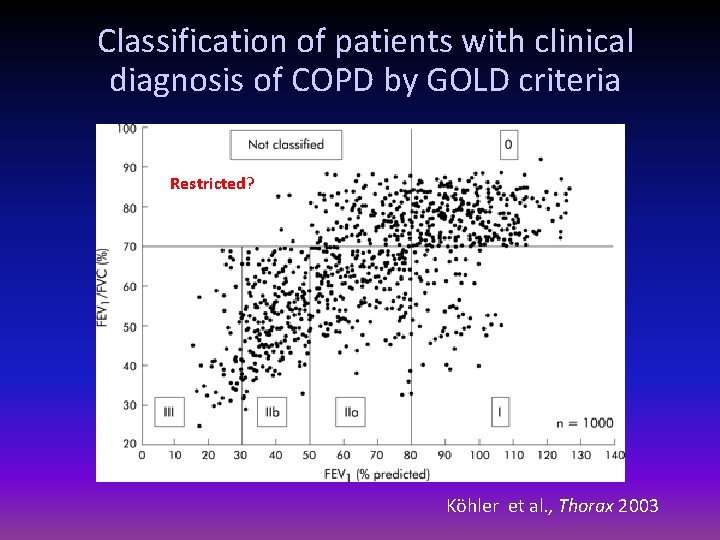
Classification of patients with clinical diagnosis of COPD by GOLD criteria Restricted? Köhler et al. , Thorax 2003

Criteria: Restrictive Disease • FEV 1: normal or mildly reduced • FVC: < 80% predicted • FEV 1/FVC: > 0. 7

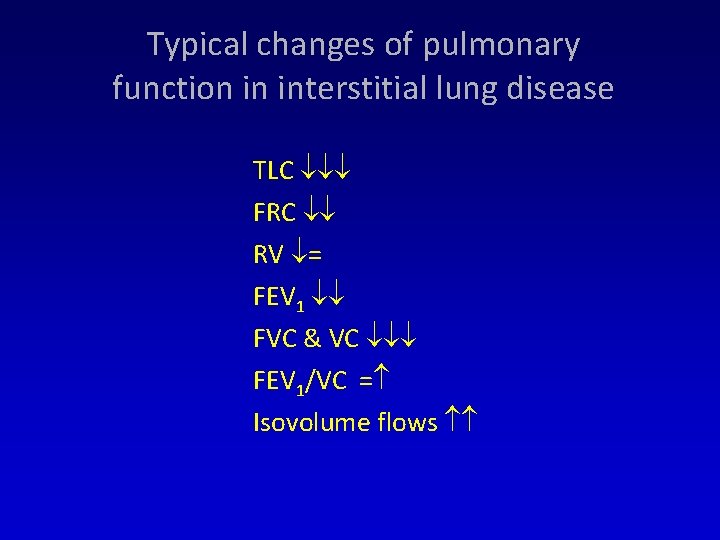
Typical changes of pulmonary function in interstitial lung disease TLC FRC RV = FEV 1 FVC & VC FEV 1/VC = Isovolume flows

Normal FEV 1/FVC not always excludes airflow obstruction Obstructed Restricted Pellegrino et al. ERJ 2005

GOLD 2007 severity classification of COPD

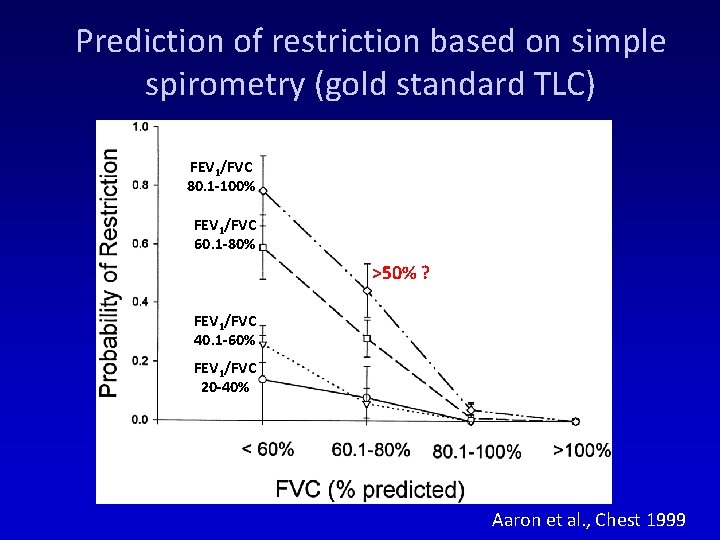
Prediction of restriction based on simple spirometry (gold standard TLC) FEV 1/FVC 80. 1 -100% FEV 1/FVC 60. 1 -80% >50% ? FEV 1/FVC 40. 1 -60% FEV 1/FVC 20 -40% Aaron et al. , Chest 1999

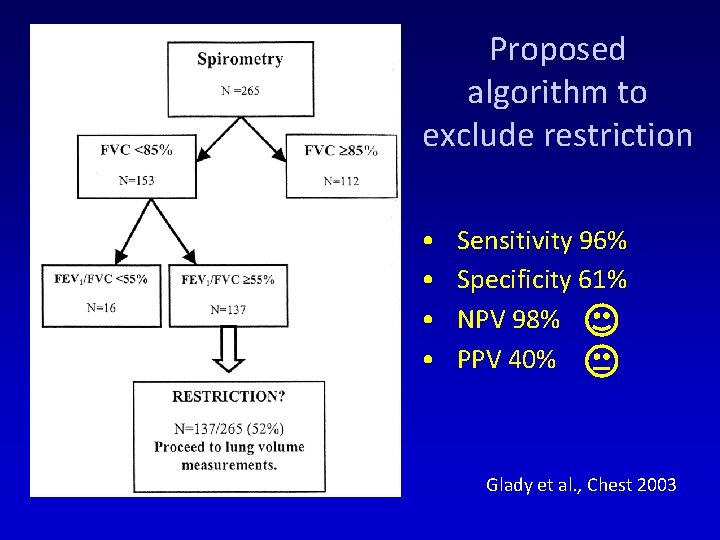
Proposed algorithm to exclude restriction • • Sensitivity 96% Specificity 61% NPV 98% PPV 40% Glady et al. , Chest 2003

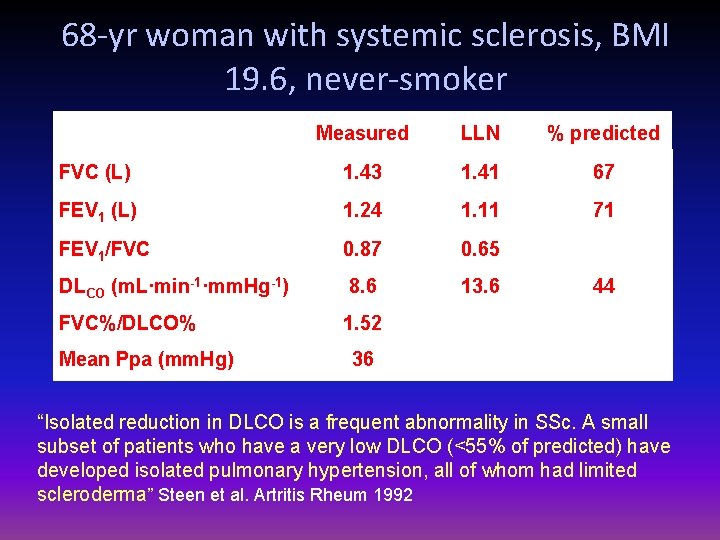
68 -yr woman with systemic sclerosis, BMI 19. 6, never-smoker Measured LLN % predicted FVC (L) 1. 43 1. 41 67 FEV 1 (L) 1. 24 1. 11 71 FEV 1/FVC 0. 87 0. 65 DLCO (m. L·min-1·mm. Hg-1) 8. 6 13. 6 FVC%/DLCO% 1. 52 Mean Ppa (mm. Hg) 44 36 “Isolated reduction in DLCO is a frequent abnormality in SSc. A small subset of patients who have a very low DLCO (<55% of predicted) have developed isolated pulmonary hypertension, all of whom had limited scleroderma” Steen et al. Artritis Rheum 1992

68 -yr woman with systemic sclerosis dyspnea, BMI 19. 6, never-smoker Measured LLN % predicted TLC (L) 2. 06 3. 32 48 FRC (L) 1. 27 1. 68 51 RV (L) 0. 63 1. 28 34

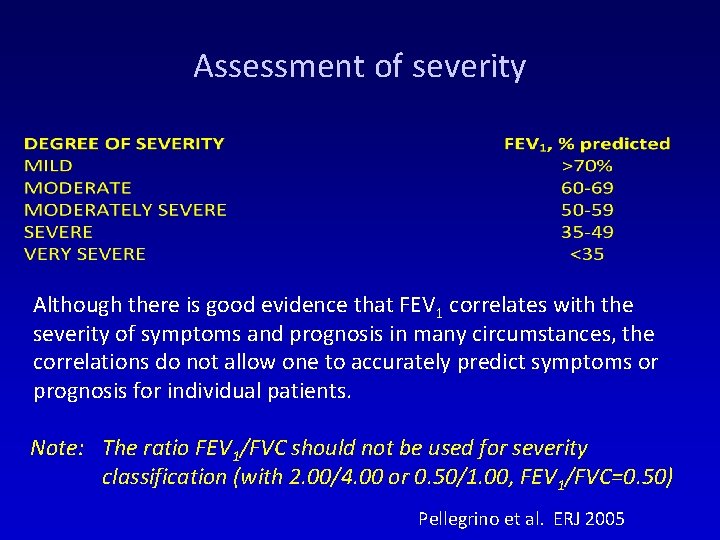
Assessment of severity Although there is good evidence that FEV 1 correlates with the severity of symptoms and prognosis in many circumstances, the correlations do not allow one to accurately predict symptoms or prognosis for individual patients. Note: The ratio FEV 1/FVC should not be used for severity classification (with 2. 00/4. 00 or 0. 50/1. 00, FEV 1/FVC=0. 50) Pellegrino et al. ERJ 2005

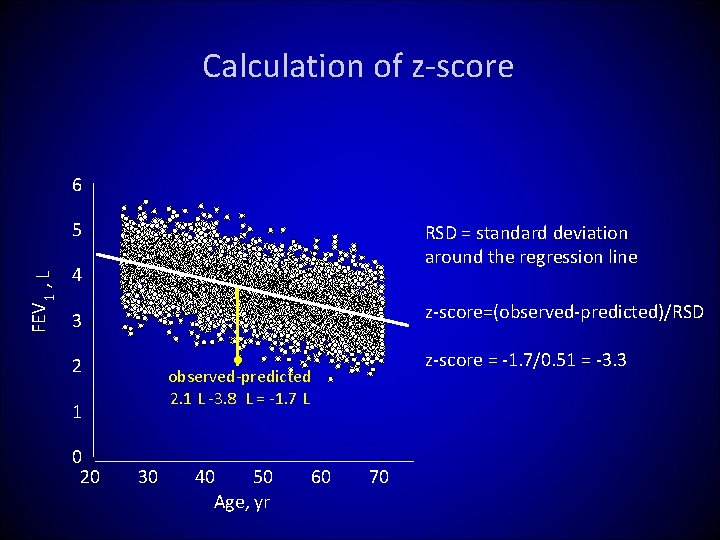
Calculation of z-score 6 FEV 1 , L 5 RSD = standard deviation around the regression line 4 z-score=(observed-predicted)/RSD 3 1 0 20 z-score = -1. 7/0. 51 = -3. 3 ● observed-predicted 2. 1 L -3. 8 L = -1. 7 L 2 30 40 50 Age, yr 60 70

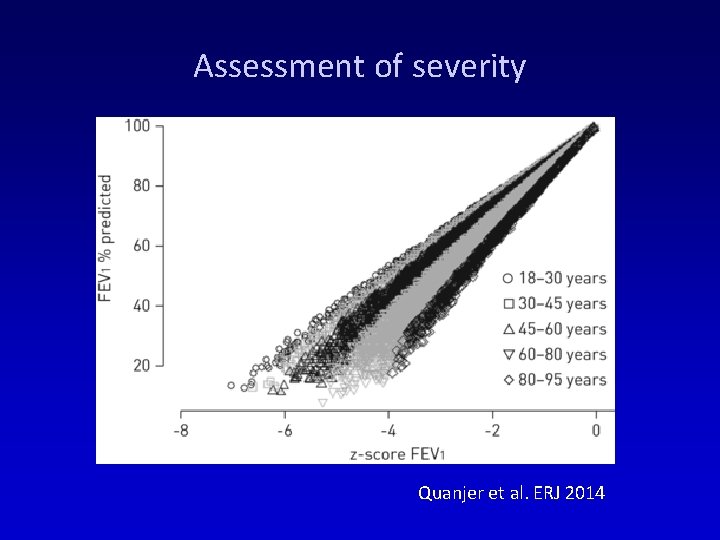
Assessment of severity Quanjer et al. ERJ 2014

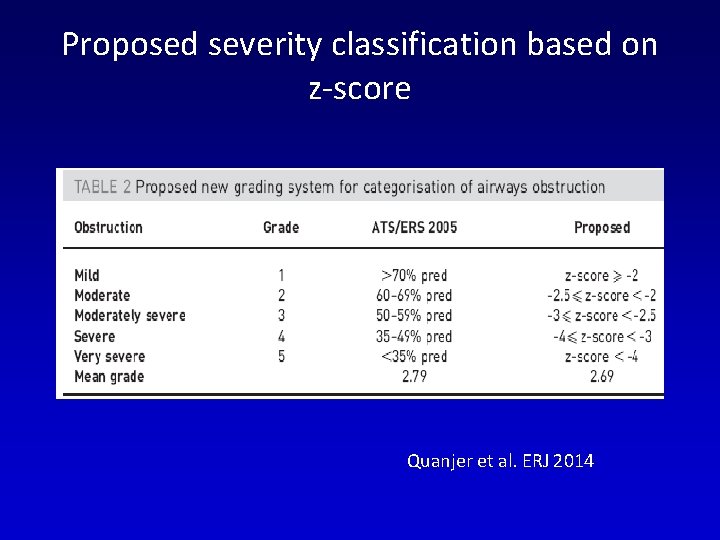
Proposed severity classification based on z-score Quanjer et al. ERJ 2014

FEV 1 in relation to severity stratification by clinically meaningful z-score thresholds z ≥-1. 64 z <-1. 64, ≥-2. 55 z <-2. 55 Vaz Fragoso et al. AJRCCM 2016

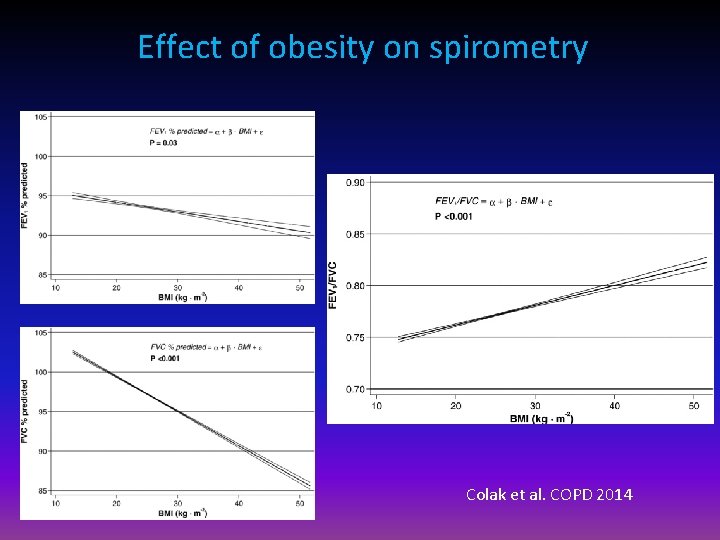
Effect of obesity on spirometry Colak et al. COPD 2014

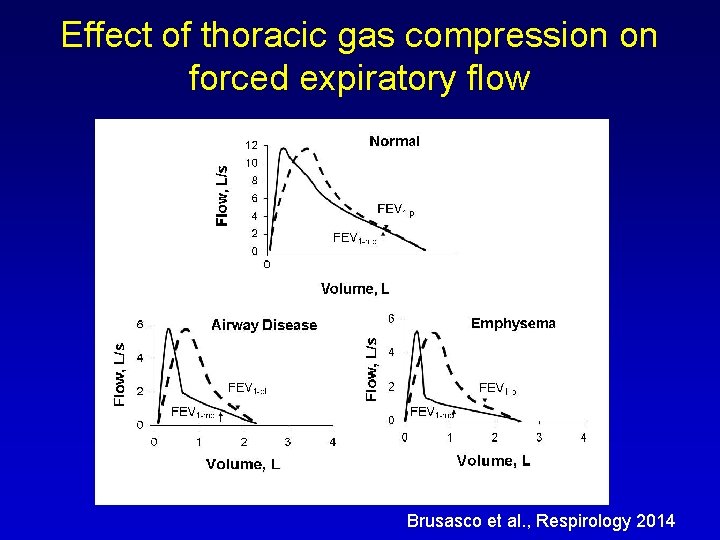
Effect of thoracic gas compression on forced expiratory flow Brusasco et al. , Respirology 2014

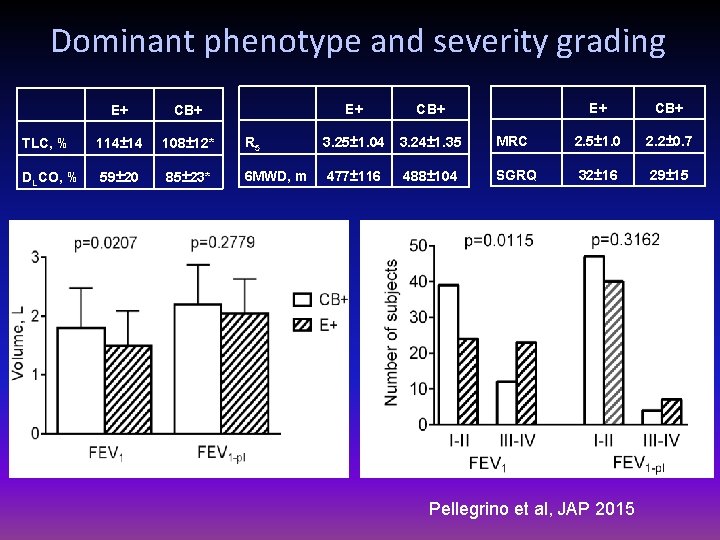
Dominant phenotype and severity grading E+ CB+ TLC, % 114± 14 108± 12* DLCO, % 59± 20 85± 23* E+ CB+ R 5 3. 25± 1. 04 3. 24± 1. 35 6 MWD, m 477± 116 488± 104 E+ CB+ MRC 2. 5± 1. 0 2. 2± 0. 7 SGRQ 32± 16 29± 15 Pellegrino et al, JAP 2015

Conclusions • Simple spirometry may give either falsely positive or falsely negative diagnosis of obstructive disorders. • Lung function abnormalities must be defined based on suitable predicting equations, not arbitrary criteria. • Measurement of lung volumes and DLCO are often necessary for correct diagnosis. • Comprehensive pulmonary function tests, radiology and clinical data allow patients’ phenotyping. • Severity classification is variably affcted by choice of indices and disease phenotype.
 Mogans cevicheria
Mogans cevicheria Sci-panss
Sci-panss Neo pi r
Neo pi r Stare de nutritie
Stare de nutritie Scor mmse interpretare
Scor mmse interpretare Scala bprs interpretare
Scala bprs interpretare Raport interpretare chestionare
Raport interpretare chestionare Testul desenul persoanei interpretare
Testul desenul persoanei interpretare Interpretare chestionar bullying
Interpretare chestionar bullying Interpretare abas 2
Interpretare abas 2 Sistema determinato indeterminato impossibile
Sistema determinato indeterminato impossibile Come mi chiamo come mi chiamo
Come mi chiamo come mi chiamo Come thou fount come thou king lyrics
Come thou fount come thou king lyrics Come home come home jesus is calling
Come home come home jesus is calling Drink in past participle
Drink in past participle E m m a n u e l
E m m a n u e l Subject + have/has + past participle
Subject + have/has + past participle Come mi vedono gli altri e come mi vedo io
Come mi vedono gli altri e come mi vedo io Come holy spirit come inflame our souls with love
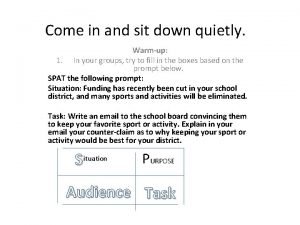
Come holy spirit come inflame our souls with love Come in come in and sit down
Come in come in and sit down Come mi chiamo come mi chiamo
Come mi chiamo come mi chiamo Come on come on turn your radio on
Come on come on turn your radio on Hey come on out shinichi hoshi
Hey come on out shinichi hoshi Come in come in and sit down
Come in come in and sit down Come lord jesus come and be born in our hearts lyrics
Come lord jesus come and be born in our hearts lyrics How to prove rational numbers are countable
How to prove rational numbers are countable 4-1 congruent figures answer key
4-1 congruent figures answer key Divergence theorem
Divergence theorem Are the two triangles similar? how do you know?
Are the two triangles similar? how do you know? Converse of parallel lines theorem
Converse of parallel lines theorem Worksheet section 3-2 angles and parallel lines
Worksheet section 3-2 angles and parallel lines State and prove hilbert basis theorem
State and prove hilbert basis theorem Proof by contraposition
Proof by contraposition Contradiction
Contradiction Prove strutturate
Prove strutturate Prove parallele scuola media
Prove parallele scuola media Prove induction
Prove induction Prove meccaniche sui metalli
Prove meccaniche sui metalli Varignon's theorem is used to find
Varignon's theorem is used to find Embedding quotes into your writing worksheet answers
Embedding quotes into your writing worksheet answers Alternate segment theorem proof
Alternate segment theorem proof Subsets definition
Subsets definition Given ac=ab+ab prove ab=bc
Given ac=ab+ab prove ab=bc Prove strutturate
Prove strutturate Esame qualifica professionale prove strutturate
Esame qualifica professionale prove strutturate Cumulative probability distribution
Cumulative probability distribution Prove invalsi 2010
Prove invalsi 2010 Myhill nerode theorem
Myhill nerode theorem Surjective function example
Surjective function example Heather must prove this theorem
Heather must prove this theorem State and prove bernoulli's principle
State and prove bernoulli's principle State and prove lagrange's theorem
State and prove lagrange's theorem Invalsi practice test for english oxford
Invalsi practice test for english oxford Lagrange's theorem formula
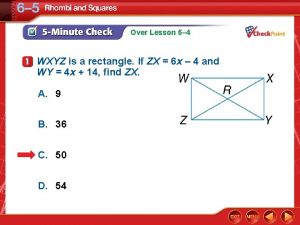
Lagrange's theorem formula Wxyz is a rectangle
Wxyz is a rectangle Proving triangle congruence
Proving triangle congruence Cook’s theorem
Cook’s theorem Celi 2 prova orale esempi
Celi 2 prova orale esempi Prova orale tfa sostegno cosa chiedono
Prova orale tfa sostegno cosa chiedono Fiaba inventata con prove da superare
Fiaba inventata con prove da superare Inclusion exclusion principle
Inclusion exclusion principle