Metrics Significant Figures Conversions and Density Review YEAH






































- Slides: 40

Metrics, Significant Figures, Conversions, and Density Review! YEAH!

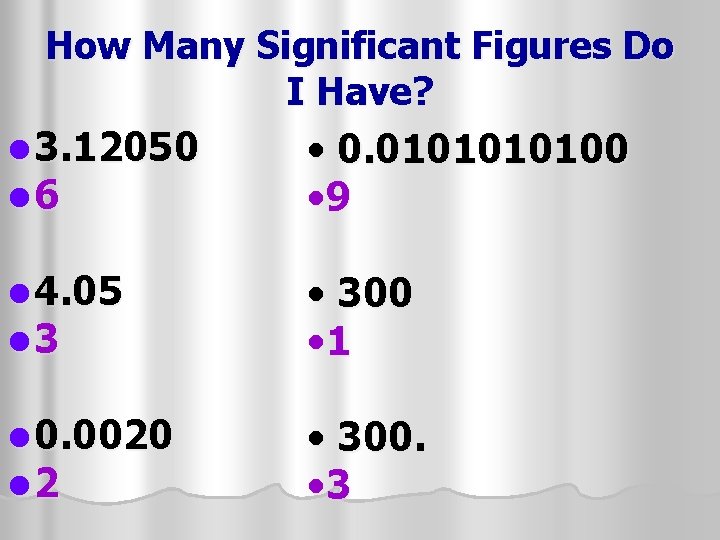
How Many Significant Figures Do I Have? l 3. 12050 l 6 • 0. 010100 • 9 l 4. 05 l 3 • 300 • 1 l 0. 0020 l 2 • 300. • 3

Question Two l 457 km = ____ cm l. Move decimal 5 to the right: 45, 700, 000

Question Three l 14. 6 yards = ____ ft l 14. 6 yds x 3 ft/1 yd = 43. 8 ft

Question Four l. Add, using correct significant figures 3. 492 + 0. 303 + 30. 30 l 34. 10

Question Five l 37, 854, 120 m. L = ______ L l. Move decimal 3 to the left, 37854. 12 No zero because of sig figs

Question 6 l. If the mass of an object is 124. 589 grams and the 3 density is 2. 45 g/cm , what is the volume of the object? l 124. 589/2. 45 = 50. 9 3 cm

Question 7 l 30, 833 mg = ____ kg l. Move decimal six to the left l 0. 030833 kg

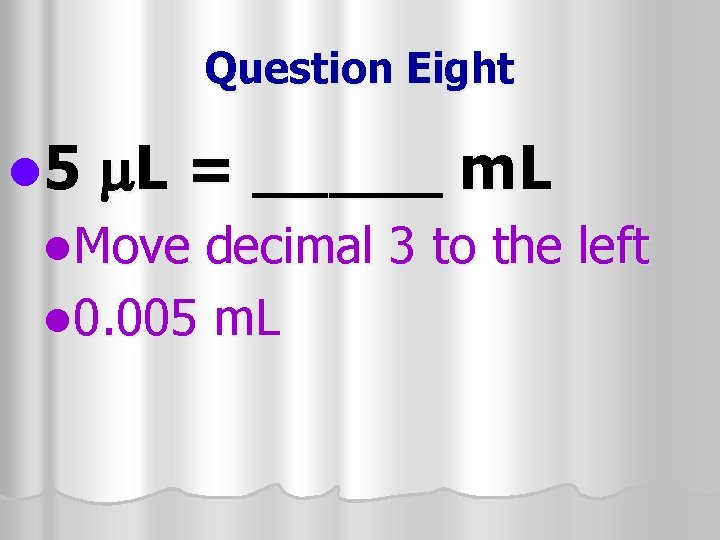
Question Eight l 5 m. L = _____ m. L l. Move decimal 3 to the left l 0. 005 m. L

Question Nine l. The density of an object 3 is 1. 56 g/cm. What is the mass of this object when the volume is 12 L? l. Convert 12 L m. L l 1. 56 x 12, 000 = 19, 000 g

Question Ten l 1. 25 s l 1. 25 days = _______ days x 24 hours x 60 min x 60 sec = 108000 s

Question Eleven l. If I am measuring with a ruler in centimeters, how many digits past the decimal should I record? l 2

Question Twelve l. How do I find the volume of a rectangular solid? l. L x. Wx. H

Question Thirteen l. I took the mass of a block of wood. The mass was 198 mg. I then measured the block and it was 14 cm wide, 3 cm high, and 17 cm long. What is the density of the wood? l. Multiply L x W x H to get volume l 0. 198/714 = 0. 0003 g/cm 3

Question Fourteen 2 km 2 m l 160 = _____ l. Normally you would move three to the right, but since it’s squared you’d do that twice… so 6 to the right l 1. 6 x 108 m 2

Question Fifteen 3 cm 3 m l 156 = _____ l. Normally you would move two to the left, but since it’s cubed, you do that three times, so move six to the left l 1. 56 x 10 -4 m 3

Question Sixteen l. One of the largest gold nuggets ever discovered was found in Australia in 1872. The nugget had a mass of 286 kg. Given that 19. 32 g/cm 3 is the density of gold, calculate the volume of this nugget l. Convert 286 kg 286000 g l 286000 / 19. 32 = 14800 cm 3

Question Seventeen l. I am measuring the length of a stirring rod and I get: 1. 25 cm, 12. 8 mm, and 0. 129 m. The actual length of the stirring rod is 19. 3 cm. Am I accurate? Am I precise? l You are not accurate because you did not get the correct number. You are also not precise because all measurements are not close to each other when converted.

Question Eighteen l. I am measuring the mass of sodium chloride for an experiment. I obtain a measurement of 12. 236 m. L. What is wrong with the way I have reported this value? l. Mass should be in units of grams, not milliliters

Question Nineteen l 48°C = _____°F l°F = 9/5°C + 32 l 9/5*48+32 = 120°F

Question Twenty l 115°F = ______ K l°C = 5/9(°F -32) l 5/9(115 -32) = 46. 1°C l. K = °C + 273 l 46. 1 + 273 = 319 K

Question Twenty - One l. Express 125, 000, 000 in scientific notation l 1. 25 x 1011

Question Twenty Two l. Express 0. 0000008 in scientific notation l 8 x 10 -7

Question Twenty Three l. What is 4. 869 x decimal notation? l 486, 900 5 10 in

Question Twenty Four l. What is 5. 21 x in decimal notation? l 0. 000521 -4 10

Question Twenty Six l 298 K = ____°F l 298 K-273 = 25°C l 9/5(25)+32 = 77. 0°F

Question Twenty seven l. How many significant figures do I have? 12, 000 l 2

Question Twenty Eight l. How many significant figures do I have? 10. 0 l 3

Question Twenty Nine l. A sample has a volume of 12. 5 L and a mass of 25. 8 kg. What is its density in g/L? l 25, 800/12. 5 = 2060 g/L

Question Twenty Nine l. A student finds the density of carbon monoxide to be 3 1. 62 g/cm. The accepted 3 value is 1. 12 g/cm. What is the percent error? l|1. 12 -1. 62| / 1. 12 x 100 = 44. 6%

Question Thirty l. One inch = 2. 54 cm l 15. 869 3 in l 15. 869 3 cm = ____ cm x 1/2. 54

Question Thirty One l. If I am driving in a car at 65 miles/hour, what is my speed in meters/second l 1 mile = 1. 6 km l 65 miles x 1. 6 km/mile x 1000 m/km x 1 hr/60 min x 1 min/60 sec = 29 m/s

Question Thirty Two l. I am measuring the mass of calcium chloride for an experiment. I measure the mass three times, obtaining values of 125. 46 g, 0. 12455 kg, 124, 999 mg. The actual mass is 1. 25 x 108 mg. Am I accurate? Am I precise? l. You are both accurate and precise

Question Thirty Three l. I measure the volume of a liquid to be 124. 56 m. L. The actual volume is 12. 8 c. L. What is my percent error? l. Convert to the same unit first! l|12. 8 – 12. 456| / 12. 8 x 100 = 2. 69%

Question Thirty Four l. Convert 6. 5 x 7 10 nm to cm. l. Nano to centi is seven to the left l 6. 5 cm

Question Thirty Five 2 mm 2 dm l 87 = ____ lmm to dm is usually two steps to the left. Since it’s squared, you’ll move four spots to the left. l 0. 0087

Question Thirty Six l. Add, figs using proper sig l 5. 0385 l 28. 1 + 8. 9 + 14. 203 =

Question Thirty Seven l. The density of an object 3 is 5. 46 g/cm. Calculate the volume, in liters, of an object that has a mass of 540 mg. l 9. 9 x 10 -5 L

Question Thirty Eight l. The mass of an object is 65 dg. The density of the object is 4. 392 g/L. What is the volume of the object in m. L? l 1500 m. L

l. Don’t forget to study your equipment and reading equipment!!