Metric System Simple Consistent Measurement up to 1790


















- Slides: 27

Metric System Simple & Consistent

Measurement up to 1790: not a pretty picture! measurement requires a recognizable standard for all but… until 1790’s every region had own standards

Standards • “standard: something used as comparison for measuring” – standard must: • be available for everyone to use • be something in nature that is same everywhere • never vary

1585 – Simon Stevin • introduced use of decimals in Europe – to allow for parts of a whole • predicted universal introduction of decimal coinage, measures and weights

1670 – Gabriel Mouton • 1 st to propose decimal system of measurement based on size of earth • Earth-based standard: 1 of longitude Earth is standard available to everyone! What an idea!

Systeme International (SI) • based on metric system • invented in 1790* – originally, earth-based standards – volume & mass linked to length – larger & smaller multiples of each unit related by powers of 10 *updated every few years (major changes in 1960 and 1991)

1790 – French Academy of Sciences created the metric system 3 Requirements

#1 Basic Standard = Earth • unit of length is portion of Earth's circumference

#2 Internal Consistency • units for capacity (volume/space) and mass related to unit of length

#3 Ease of Use - Calculations • larger and smaller units created by multiplying or dividing basic units by factors of 10

Fundamental (Base) Units • based on object/event in nature • SI system has 7 fundamental units • probably already know 4 of them • any guesses as to which ones you know?

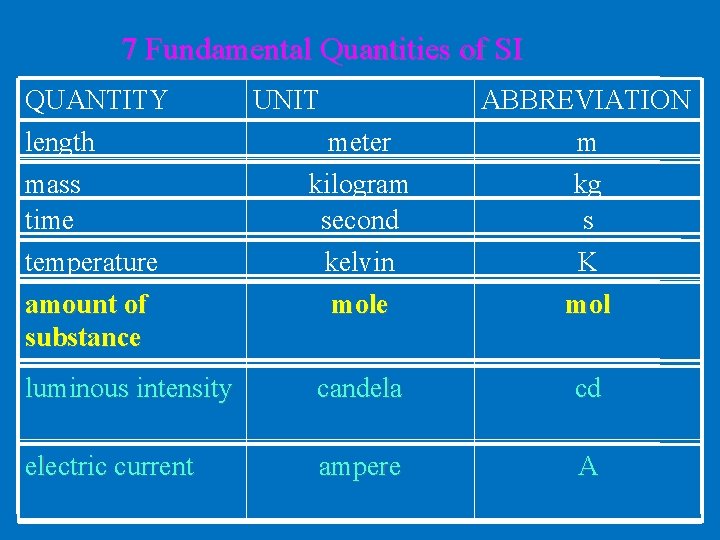
7 Fundamental Quantities of SI QUANTITY length meter ABBREVIATION m kilogram second kelvin mole kg s K mol luminous intensity candela cd electric current ampere A mass time temperature amount of substance UNIT

Derived Units • combinations of fundamental units • examples: – speed (meters/second) – area (length x width) – volume (length x width x height) – density (mass / volume)

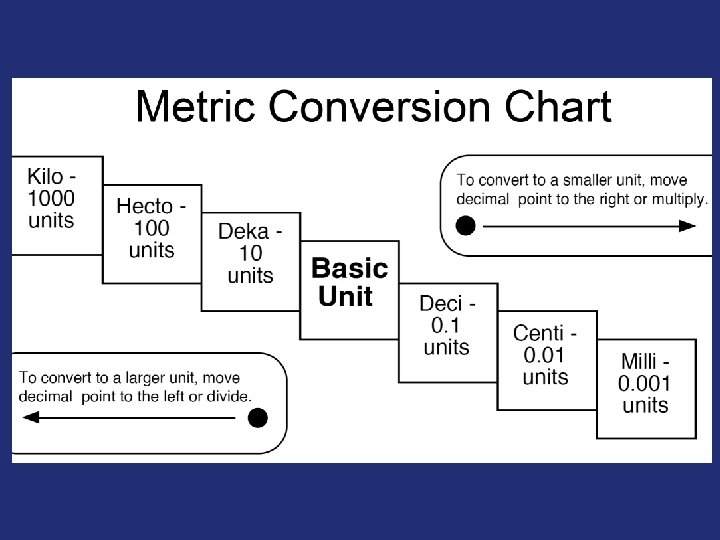
Ease of Use - Names larger & smaller multiples of same unit named by series of prefixes relating to base unit

smaller units • 1/10 of meter = decimeter (dm) • 1/100 of meter = centimeter (cm) • 1/1000 of meter = millimeter (mm) larger units • 10 meters = dekameter (dam) • 100 meters = hectometer (hm) • 1000 meters = kilometer (km)


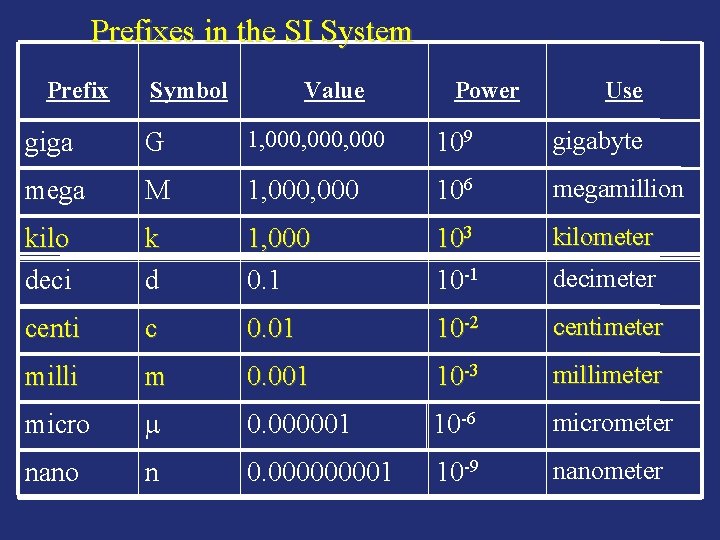
Prefixes in the SI System Prefix Symbol Value Power Use giga G 1, 000, 000 109 gigabyte mega M 1, 000 106 megamillion kilo deci k d 1, 000 0. 1 103 10 -1 kilometer centi c 0. 01 10 -2 centimeter milli m 0. 001 10 -3 millimeter micro 0. 000001 10 -6 micrometer nano n 0. 00001 10 -9 nanometer decimeter

Prefixes • used for all 7 fundamental units! – kilometer – milliliter – centigram – microsecond – nanokelvin

1790 - Jefferson • proposed decimal-based measurement system for US cons: no prefixes & too many names

1792 – U. S. Mint • produced world’s first decimal currency (one dollar = 100 cents) • • 100 cents in dollar 4 quarters in dollar 10 dimes in dollar 20 nickels in dollar can see how this can be confusing: - names not related to each other - no consistency with parts of dollar

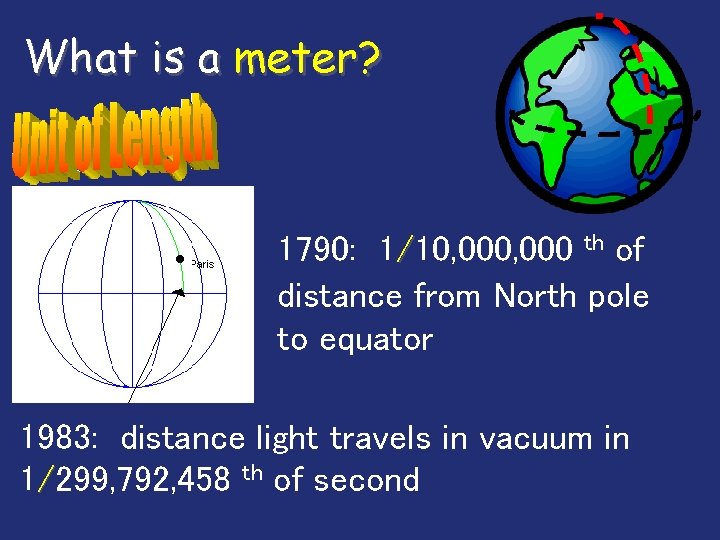
What is a meter? 1790: 1/10, 000 th of distance from North pole to equator 1983: distance light travels in vacuum in 1/299, 792, 458 th of second

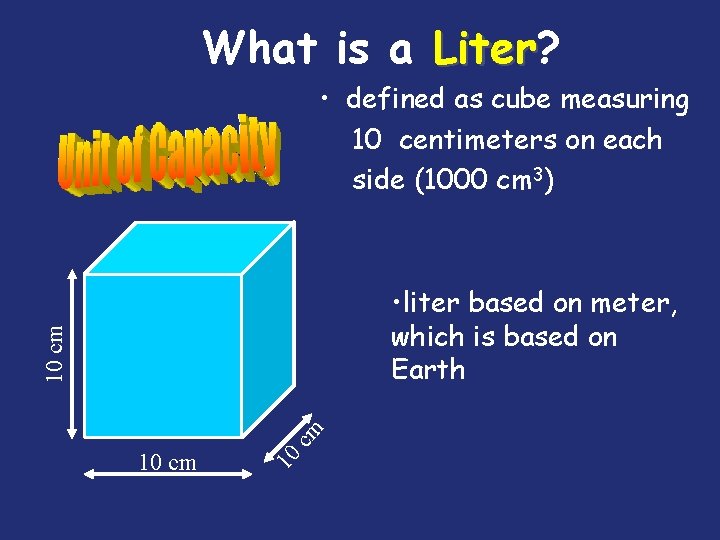
What is a Liter? Liter • defined as cube measuring 10 centimeters on each side (1000 cm 3) 10 10 cm cm 10 cm • liter based on meter, which is based on Earth

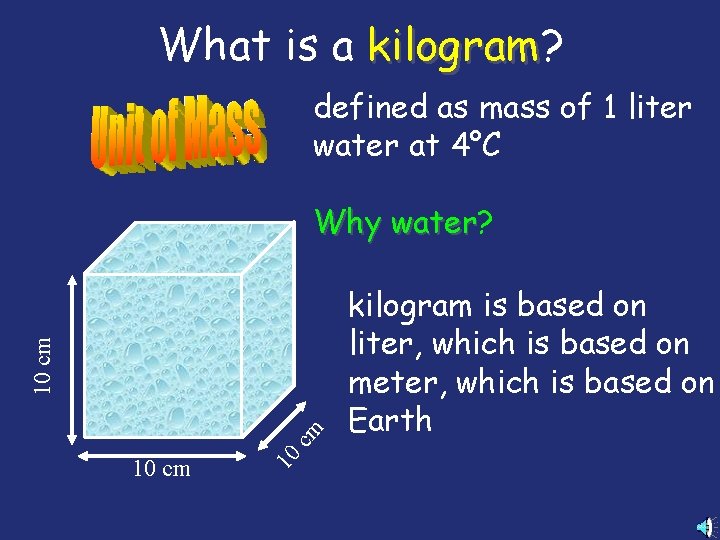
What is a kilogram? kilogram defined as mass of 1 liter water at 4°C 10 cm Why water? water kilogram is based on liter, which is based on meter, which is based on Earth

What is a second? second originally defined as 1/86, 400 th of average solar day defined now in terms of electron transitions in Cs 133

What is a Kelvin? Kelvin is defined in terms of water & absolute zero 0 K = Absolute zero bp of H 2 O = 100�C = 373 K mp of H 2 O = 0�C = 273 K

What is a mole? mole amount any substance that has as many particles as # atoms found in 0. 012 kg of carbon-12

prototype kilogram stored in vault in France