Metric System Measurement Measurements The English system uses



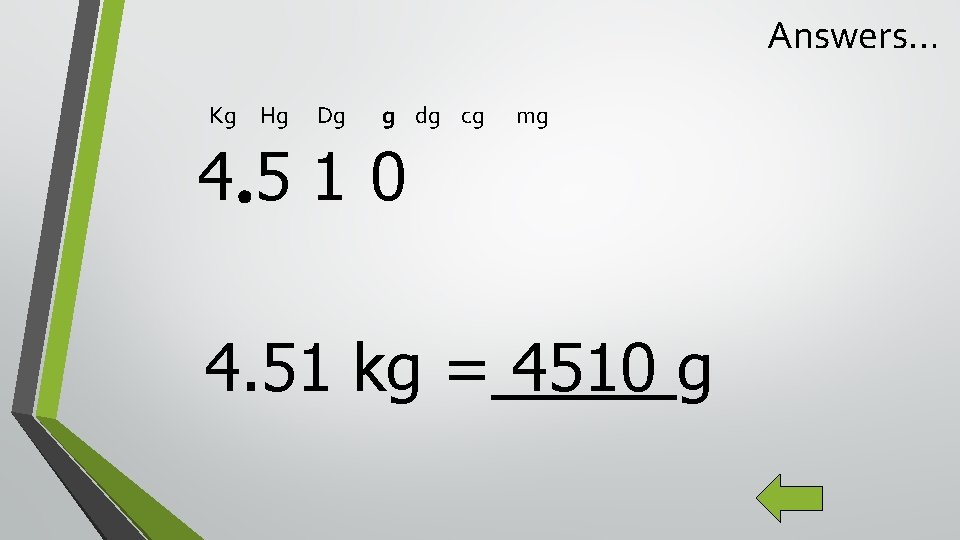







- Slides: 24

Metric System Measurement

Measurements • The English system uses measurements • like feet, yards, miles, and gallons. • Also known as the International System of Units or the SI system

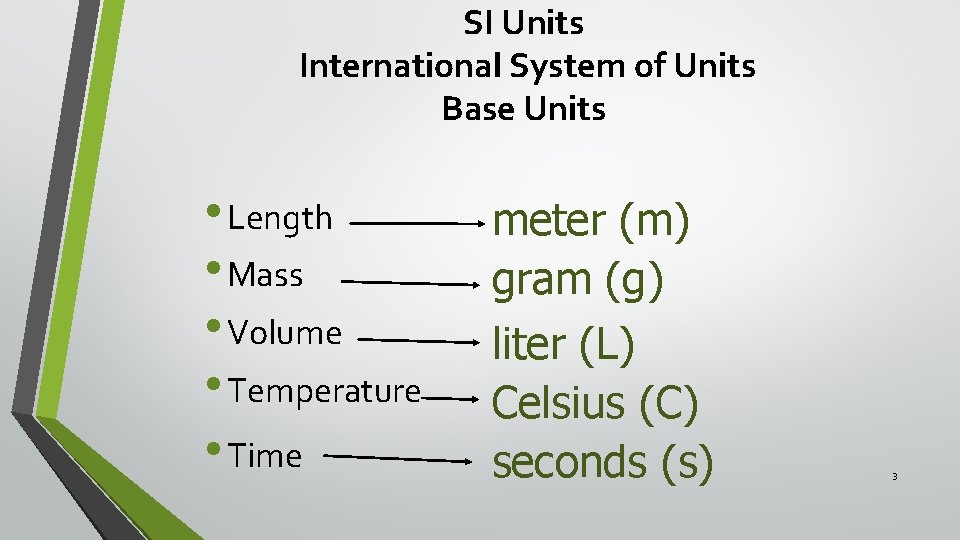
SI Units International System of Units Base Units • Length • Mass • Volume • Temperature • Time meter (m) gram (g) liter (L) Celsius (C) seconds (s) 3

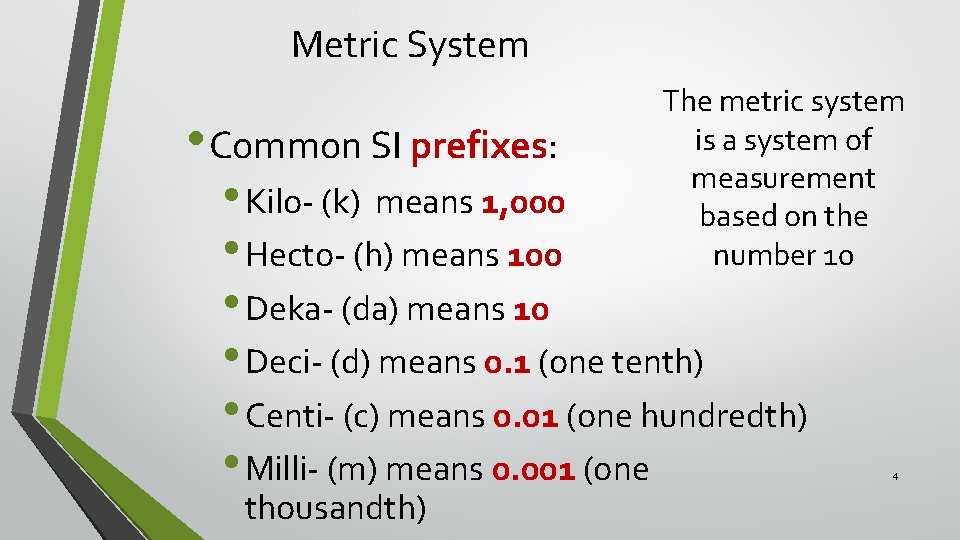
Metric System • Common SI prefixes: The metric system is a system of measurement based on the number 10 • Kilo- (k) means 1, 000 • Hecto- (h) means 100 • Deka- (da) means 10 • Deci- (d) means 0. 1 (one tenth) • Centi- (c) means 0. 01 (one hundredth) • Milli- (m) means 0. 001 (one thousandth) 4

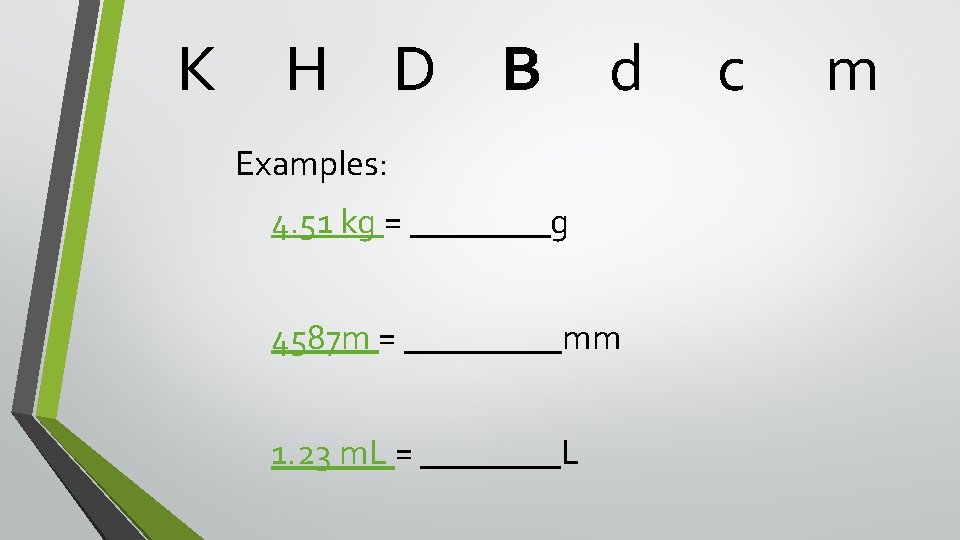
Conversions between large and small quantities K H King Henry • D B Died By d How many places to move the decimal In what direction to move the decimal What base unit to use m drinking chocolate milk This chart tells you three things: • • • c

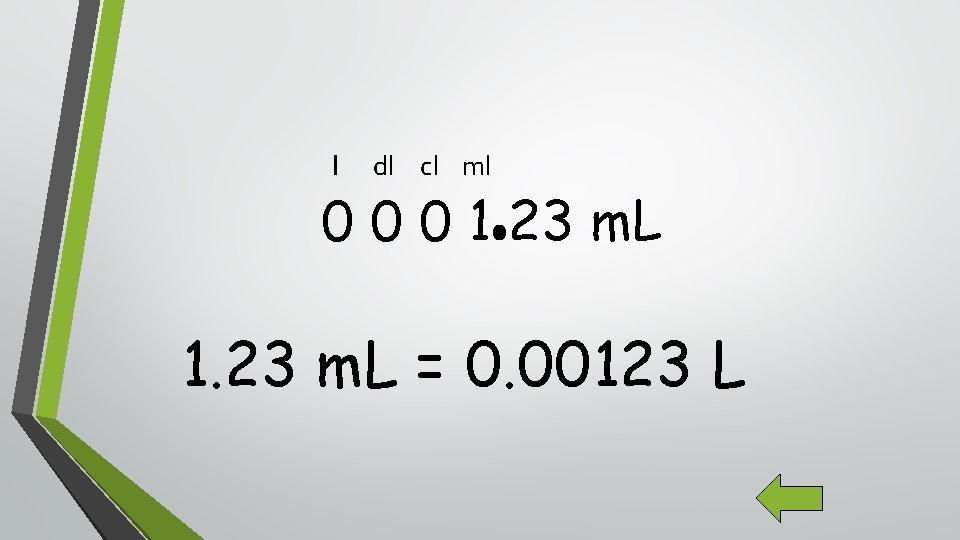
K H D B d Examples: 4. 51 kg = ____g 4587 m = _____mm 1. 23 m. L = ____L c m
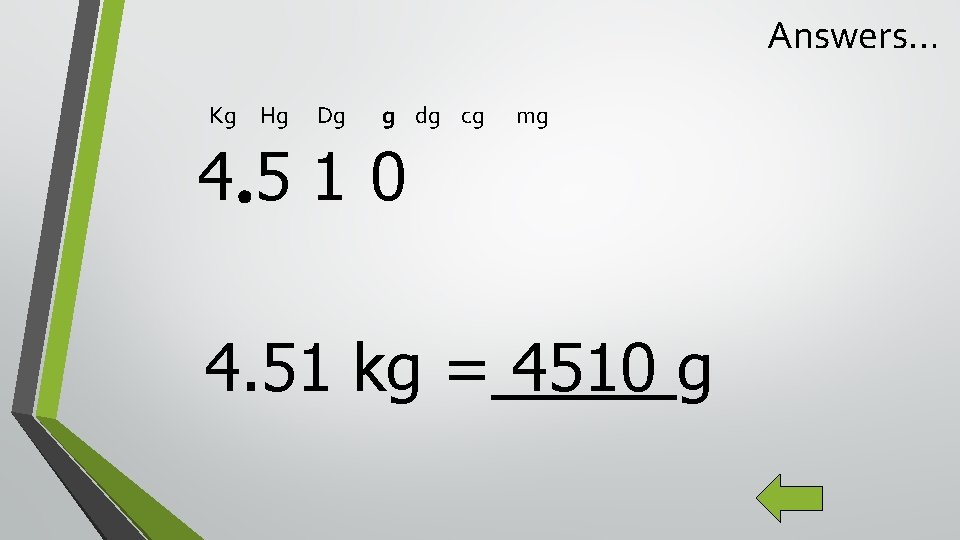
Answers… Kg Hg Dg g dg cg mg 4510 4. 51 kg = 4510 g

m dm cm mm 4587 0 0 0 4587 m = 4, 587, 000 mm

l dl cl ml 0 0 0 1 23 m. L 1. 23 m. L = 0. 00123 L

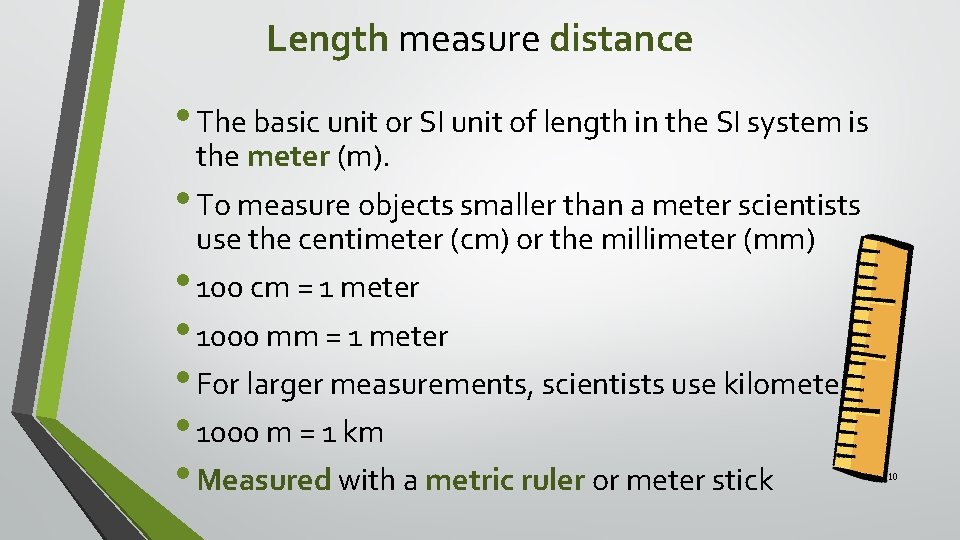
Length measure distance • The basic unit or SI unit of length in the SI system is the meter (m). • To measure objects smaller than a meter scientists use the centimeter (cm) or the millimeter (mm) • 100 cm = 1 meter • 1000 mm = 1 meter • For larger measurements, scientists use kilometers. • 1000 m = 1 km • Measured with a metric ruler or meter stick 10

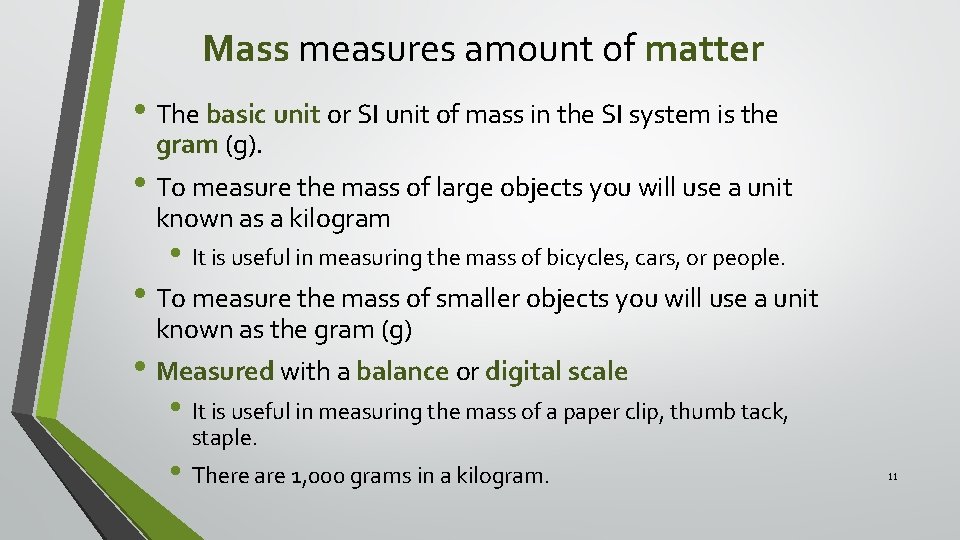
Mass measures amount of matter • The basic unit or SI unit of mass in the SI system is the gram (g). • To measure the mass of large objects you will use a unit known as a kilogram • It is useful in measuring the mass of bicycles, cars, or people. • To measure the mass of smaller objects you will use a unit known as the gram (g) • Measured with a balance or digital scale • It is useful in measuring the mass of a paper clip, thumb tack, staple. • There are 1, 000 grams in a kilogram. 11

Measuring Mass Digital Scale Triple-beam balance 12

The Difference Between Mass and Weight • Weight is a measure of the force of gravity acting on an object. • Measure weight by using a scale. When you stand on a scale gravity pulls you downward. However, if you weighed yourself on the moon you would weigh less. • Mass is a measure of how much matter an object contains, it remains constant no matter where an object may be. Your mass is the same on the moon as it is on Earth. ** The weight of an object may differ on the moon, or on earth, but the mass stays the same** 13

Measuring Volume of Liquids • Volume is the amount of space an object takes up. • To measure the volume of a liquid, you will use a base unit or SI unit known as a liter (L). • You can measure large liquid volumes in kiloliters (k. L) • You can measure smaller liquid volumes in milliliters (m. L) • 1, 000 m. L = 1 L • Measured with a graduated cylinder 14

Graduated Cylinder • Scientists commonly use a graduated cylinder to measure liquid volumes. • The graduated cylinder is marked off in milliliter segments. • When liquid is poured into a graduated cylinder, the top surface of the water is curved. This curve is called the meniscus. To determine the volume of the water, you should read the bottom of the meniscus 15

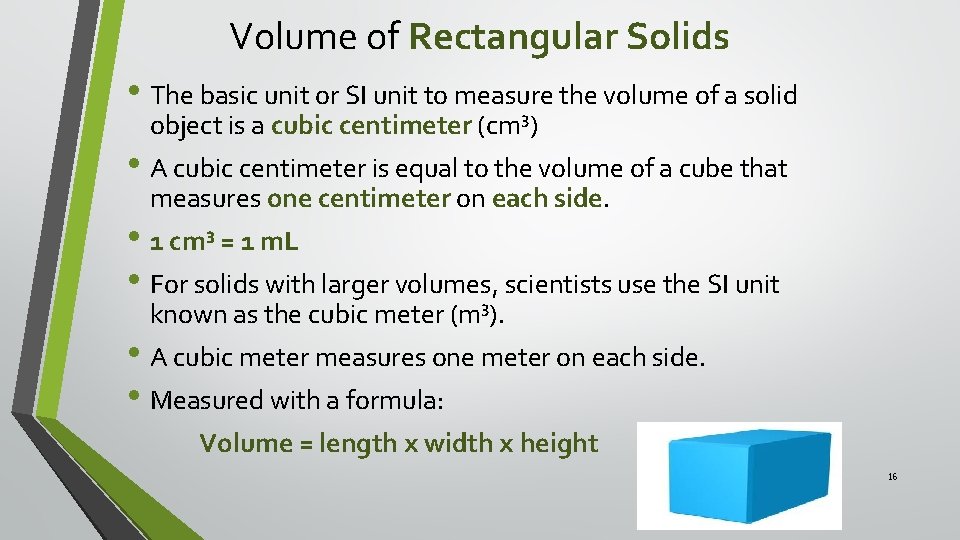
Volume of Rectangular Solids • The basic unit or SI unit to measure the volume of a solid object is a cubic centimeter (cm³) • A cubic centimeter is equal to the volume of a cube that measures one centimeter on each side. • 1 cm³ = 1 m. L • For solids with larger volumes, scientists use the SI unit known as the cubic meter (m³). • A cubic meter measures one meter on each side. • Measured with a formula: Volume = length x width x height 16

Volume of Irregular Solids • If you wanted to find the volume of an object that you could not determine the length, width, and height (example: rock), you would immerse the object in water and see how much the water level rises. • You would subtract the amount of water that you started with from the amount of water that the object forced the water to rise to and you would know the volume of your object. • Known as water displacement 17

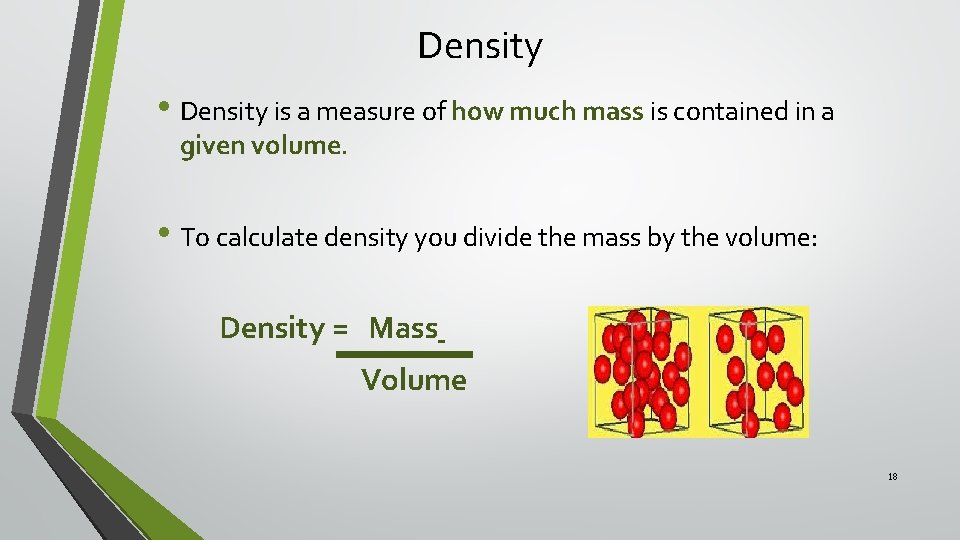
Density • Density is a measure of how much mass is contained in a given volume. • To calculate density you divide the mass by the volume: Density = Mass Volume 18

Density • Because density is actually made up of two other measurements – mass and volume - an object’s density is expressed in two units. • Two common units of density are grams per cubic centimeter (g/cm³) for regular solid items and grams per milliliter (g/m. L) for irregular solid objects. • The numerator is the measure of mass while the denominator is the measure of volume 19

Densities of Common Substances Substance Air Ice Water Aluminum Gold Density (g/cm³) 0. 001 0. 9 The density of an object stays the same 1. 0 no matter how large or 2. 7 small a sample of the 19. 3 substance is. 20

Density • An object will float if it is less dense than the surrounding liquid. • A piece of wood with the density of 0. 8 g/cm³ will float in water because the density of water is 1. 0 g/cm³ • A ring made of pure silver with a density of 10. 5 g/cm³ will sink in water because it is more dense. 21

Time • Time is the period between two events • The basic unit or SI unit of time in the SI system is the second (s). • Clocks and stopwatches are used to measure time • Common Conversions for Time • 1 s = 1, 000 ms (milliseconds) • 1 min = 60 s • 1 h = 60 min 22

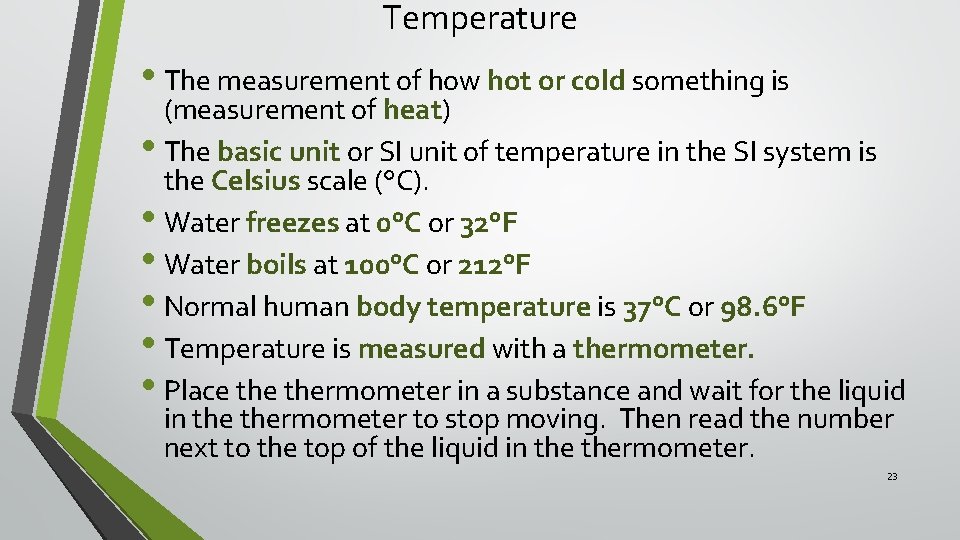
Temperature • The measurement of how hot or cold something is (measurement of heat) • The basic unit or SI unit of temperature in the SI system is the Celsius scale (°C). • Water freezes at 0°C or 32°F • Water boils at 100°C or 212°F • Normal human body temperature is 37°C or 98. 6°F • Temperature is measured with a thermometer. • Place thermometer in a substance and wait for the liquid in thermometer to stop moving. Then read the number next to the top of the liquid in thermometer. 23

Temperature Scales 24