Metric Our country still uses an old system

















- Slides: 24

Metric

Our country still uses an old system with non uniform measurements such as: fractions of an inch. . . 12 inches to a foot…. 3 feet to a yard…. 5. 5 yards to a rod. . . 320 rods to a mile. . . 43, 560 sq ft to an acre. . . But almost all other countries use the metric system, which is disadvantageous for us.

But we do use the metric for a few things: We buy cola in liters. . . We buy memory cards in bites… We run 10 km races. . . We swim in 25 meter pools. . . Why haven’t we switched entirely to metric?

Measuring Length in meters �When measuring a person we would use meters. �If we are measuring an ant, would meters still be feasible? What should we use? �If we are measuring the distance from your house to the school, what should we use? �Always pick a prefix with a value close to what you are measuring.

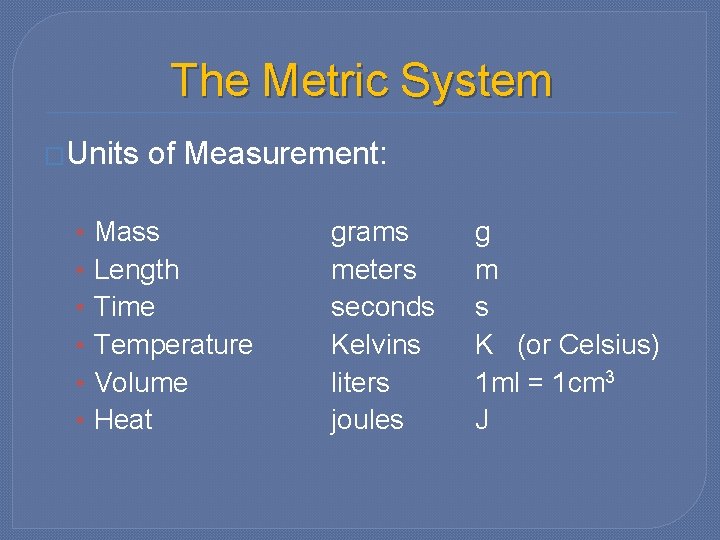
The Metric System �Units • • • of Measurement: Mass Length Time Temperature Volume Heat grams meters seconds Kelvins liters joules g m s K (or Celsius) 1 ml = 1 cm 3 J

Name the units: �Mass �Grams �Volume �Liters �Length �Meters �Temperature �Kelvin �Energy �Joules �Time �Seconds (Celcius)

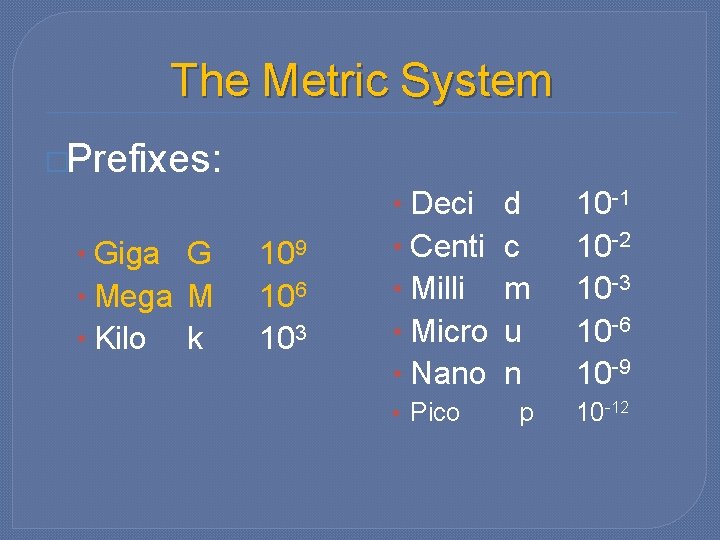
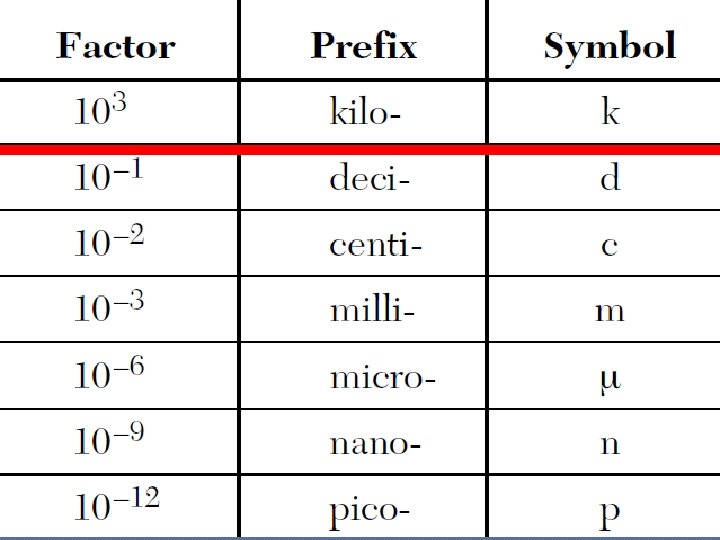
The Metric System �Prefixes: • Giga G • Mega M • Kilo k 109 106 103 • Deci • Centi • Milli • Micro • Nano • Pico d c m u n 10 -1 10 -2 10 -3 10 -6 10 -9 p 10 -12

The Metric System �If a unit is getting larger (m km) the number must get smaller. If the unit gets smaller (m cm) the number gets larger. �Examples: • 1. 23. 5 cm = km • 2. 3567 m. L = 0. 000235 L • 3. 0. 0984 g = 3. 567 ug 98400


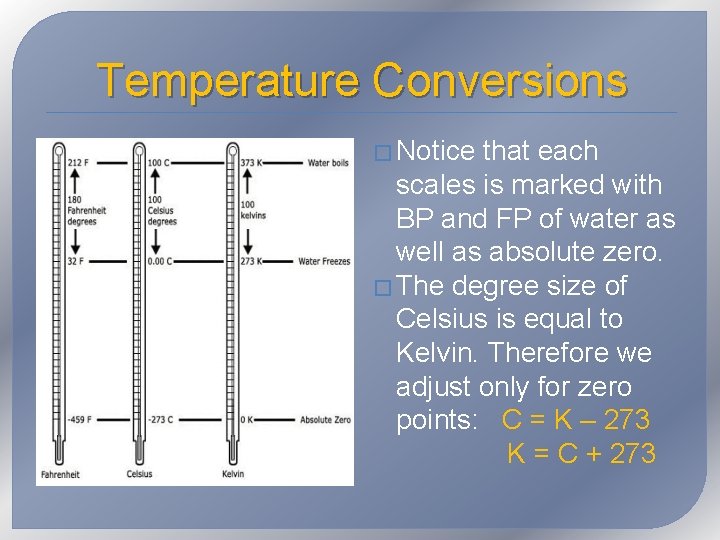
Temperature Conversions � Notice that each scales is marked with BP and FP of water as well as absolute zero. � The degree size of Celsius is equal to Kelvin. Therefore we adjust only for zero points: C = K – 273 K = C + 273

Thinking in Celsius �-10° Celsius = frigid (14° F) � 0° Celsius = cold (32° F) � 10° Celsius = cool (50° F) � 20° Celsius = comfortably warm (68° F) � 30° Celsius = hot (86° F) � 40° Celsius = very hot (104° F) � 50° Celsius = Phoenix Hot (120°F)


Density

Density depends on: �Mass: the amount of matter an object contains. (This is different than weight, which is mass plus gravity)

Density also depends on… Volume: amount of SPACE an object takes up

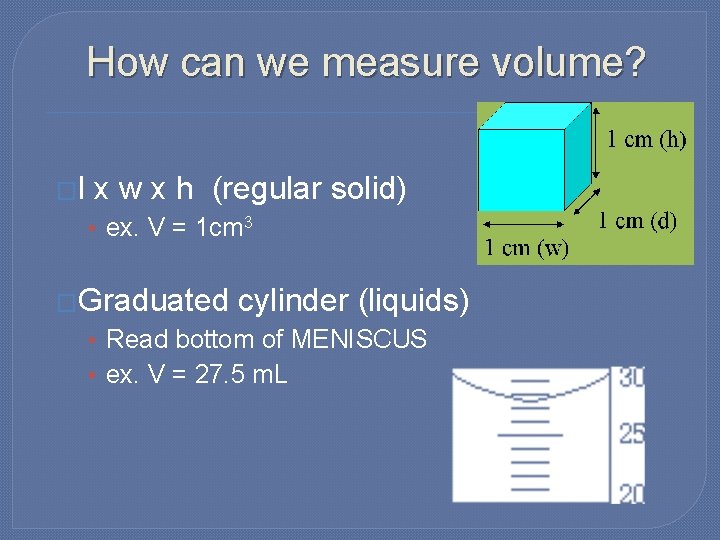
How can we measure volume? �l x w x h (regular solid) • ex. V = 1 cm 3 �Graduated cylinder (liquids) • Read bottom of MENISCUS • ex. V = 27. 5 m. L

Measuring Volume: Irregular Solid Water displacement method: 1. 2. 3. Measure initial volume Measure final volume with object The Difference is the volume of the object

Example What is the volume of the solid a. b. c. d. 46 m. L 54 m. L 8 m. L 26 m. L

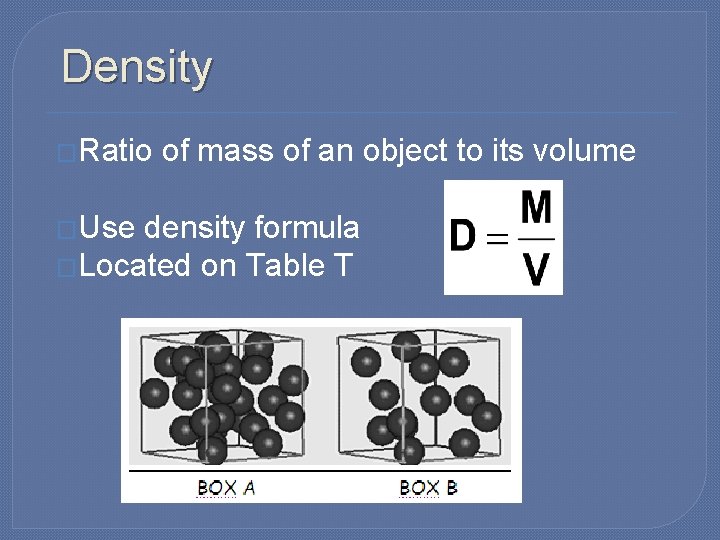
Density �Ratio �Use of mass of an object to its volume density formula �Located on Table T

Example 1 What is the density of an object with a mass of 60 g and a volume of 2 cm 3? 60/2 = 30 g/cm 3

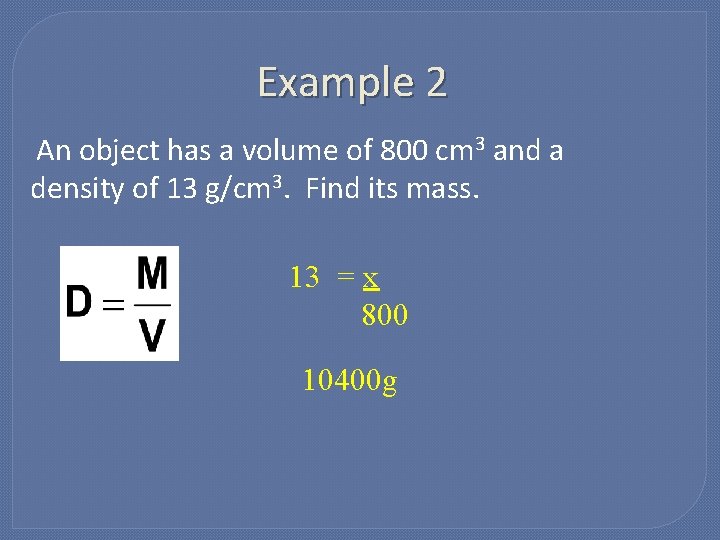
Example 2 An object has a volume of 800 cm 3 and a density of 13 g/cm 3. Find its mass. 13 = x 800 10400 g

Example 3: How to solve for mass or volume if density is not given: USE TABLE S Example: The volume of an aluminum sample is 100 cm 3. What is the mass of the sample? The density of aluminum on table S is 2. 70 g/cm 3 2. 70 = x 100 270 g

Example 4 Determine the volume of an aluminum object with a mass of 54 g. 2. 70= 54 x 20 cm 3 2. 70 x = 54

Is density constant? 1. Draw representations of liquid water when it is cold and when it is hot. A. In which condition will the particles be closer together? B. In which condition will the density be greatest? 2. Let’s describe how a Galileo thermometer works.