Metric Conversions Scientific Notation and Dimensional Analysis Scientific









- Slides: 11

Metric Conversions, Scientific Notation, and Dimensional Analysis

Scientific Notation Steps for converting: • Put the decimal after the first digit and drop the zeros Ex. 123, 000, 000 1. 23 • To find the exponent, count the number of places from the decimal to the end of the number. Ex. In 123, 000, 000 there are 11 places therefore we write it as 1. 23 x 1011 • Numbers less than 1 will have negative exponents Ex. 0. 000001 will be written as 1 x 10 -6

Examples Convert: • 0. 005 • 5, 050 • 0. 0008 • 1, 000, 000 • 0. 25 • 0. 0025 • 500 • 5, 000

International System of Units • Built on a set of seven metric units, called base units • Prefixes are added to the names of SI base units to represent quantities that are larger or smaller than the base units

SI Base Units • • Length – meter (m) Mass – kilogram (kg) Time – second (s) Temperature – Kelvin (K) Amount of substance – mole (mol) Electric current – ampere (A) Luminous intensity – candela (cd)

Metric Prefixes and Symbols • Kilo – k • Hecto – h • • • Deka – da BASE Deci – d Centi – c Milli – m King Henry Died By Drinking Chocolate


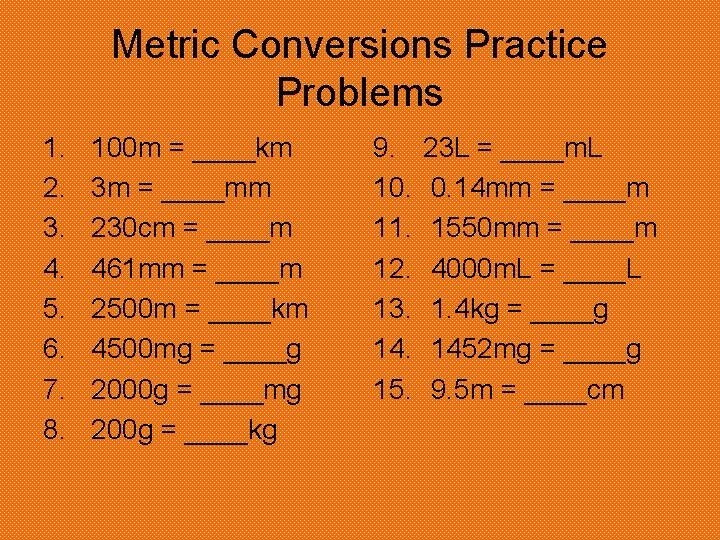
Metric Conversions Practice Problems 1. 2. 3. 4. 5. 6. 7. 8. 100 m = ____km 3 m = ____mm 230 cm = ____m 461 mm = ____m 2500 m = ____km 4500 mg = ____g 2000 g = ____mg 200 g = ____kg 9. 10. 11. 12. 13. 14. 15. 23 L = ____m. L 0. 14 mm = ____m 1550 mm = ____m 4000 m. L = ____L 1. 4 kg = ____g 1452 mg = ____g 9. 5 m = ____cm

Derived SI units • • • Area – sq. meter (m 2) Volume – cubic meter (m 3) Density – kg per cubic meter (kg/m 3) Molar mass – kg per mole (kg/mol) Concentration – moles per liter (M) Molar volume – cubic meters per mol (m 3/mol)

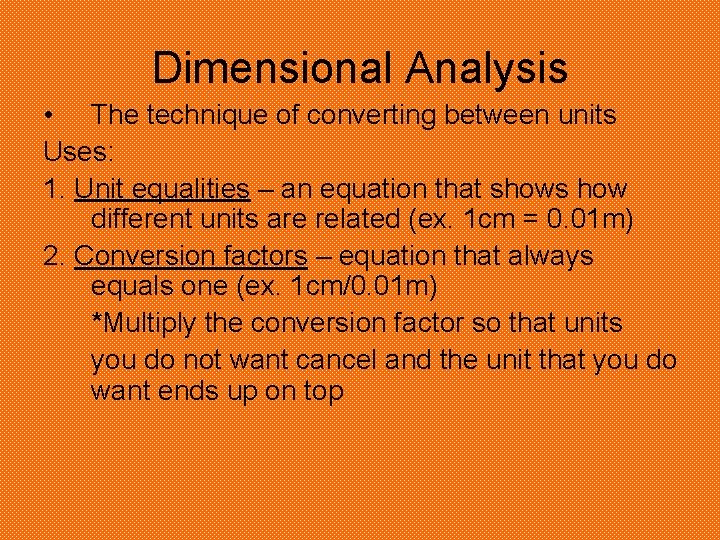
Dimensional Analysis • The technique of converting between units Uses: 1. Unit equalities – an equation that shows how different units are related (ex. 1 cm = 0. 01 m) 2. Conversion factors – equation that always equals one (ex. 1 cm/0. 01 m) *Multiply the conversion factor so that units you do not want cancel and the unit that you do want ends up on top

Homework Problems 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 64. 5 dm = ___ mm 91. 2 m = ___ km 96. 5 in = ___ yds 1. 235 ft = ___cm 8. 95 k. J = ___cal 34. 5 miles = ___m 52. 3 cm = ___ft 6. 98 m/min = ___km/hr 59. 63 cal = ___J 96. 5 k. J = ___cal 1 year = ___ sec