Methylation and Glutathione Keys to Chronic Fatigue Syndrome










































- Slides: 56

Methylation and Glutathione, Keys to Chronic Fatigue Syndrome Rich Van Konynenburg, Ph. D. Independent Researcher/Consultant richvank@aol. com Orthomolecular Health Medicine Society 14 th Annual Scientific Meeting San Francisco February 29 -March 2, 2008

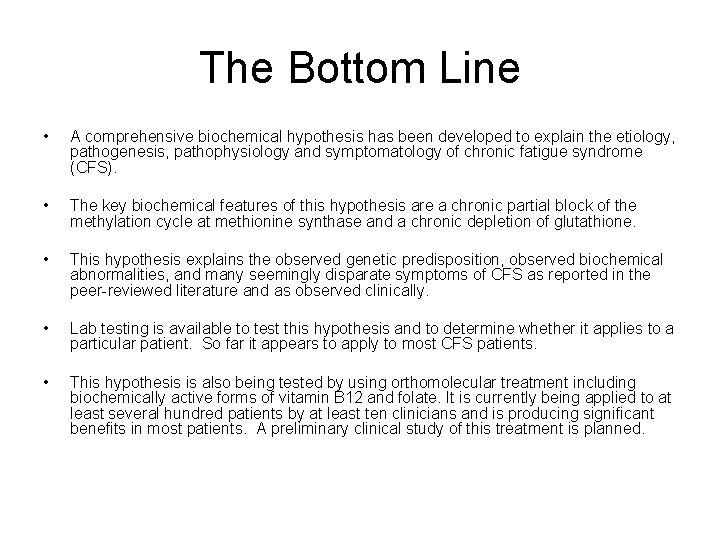
The Bottom Line • A comprehensive biochemical hypothesis has been developed to explain the etiology, pathogenesis, pathophysiology and symptomatology of chronic fatigue syndrome (CFS). • The key biochemical features of this hypothesis are a chronic partial block of the methylation cycle at methionine synthase and a chronic depletion of glutathione. • This hypothesis explains the observed genetic predisposition, observed biochemical abnormalities, and many seemingly disparate symptoms of CFS as reported in the peer-reviewed literature and as observed clinically. • Lab testing is available to test this hypothesis and to determine whether it applies to a particular patient. So far it appears to apply to most CFS patients. • This hypothesis is also being tested by using orthomolecular treatment including biochemically active forms of vitamin B 12 and folate. It is currently being applied to at least several hundred patients by at least ten clinicians and is producing significant benefits in most patients. A preliminary clinical study of this treatment is planned.

Topics to be covered • History of the Glutathione Depletion—Methylation Cycle Block (GDMCB) hypothesis • Description of glutathione • Description of the methylation cycle and associated biochemical pathways • Etiology of CFS, according to this hypothesis • Pathogenesis of CFS, according to this hypothesis • The role of genetic polymorphisms in CFS • Accounting for observed biochemical abnormalities, pathophysiology and symptoms of CFS with this hypothesis

Topics to be covered (continued) • Why is CFS more prevalent in women? • Lab testing to test this hypothesis and to determine whether it applies to a given case of CFS • Hypothesis testing using a treatment based on this hypothesis • Results of hypothesis testing to date • Some questions that remain to be answered • Planned clinical study • References

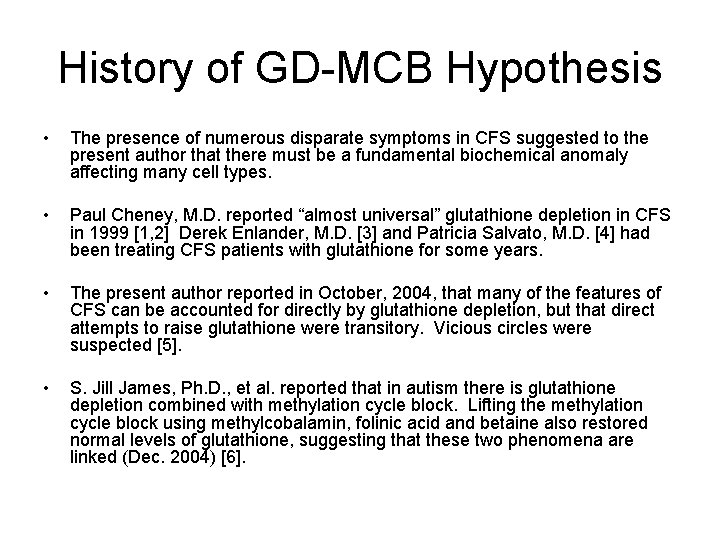
History of GD-MCB Hypothesis • The presence of numerous disparate symptoms in CFS suggested to the present author that there must be a fundamental biochemical anomaly affecting many cell types. • Paul Cheney, M. D. reported “almost universal” glutathione depletion in CFS in 1999 [1, 2] Derek Enlander, M. D. [3] and Patricia Salvato, M. D. [4] had been treating CFS patients with glutathione for some years. • The present author reported in October, 2004, that many of the features of CFS can be accounted for directly by glutathione depletion, but that direct attempts to raise glutathione were transitory. Vicious circles were suspected [5]. • S. Jill James, Ph. D. , et al. reported that in autism there is glutathione depletion combined with methylation cycle block. Lifting the methylation cycle block using methylcobalamin, folinic acid and betaine also restored normal levels of glutathione, suggesting that these two phenomena are linked (Dec. 2004) [6].

History of GD-MCB Hypothesis (continued) • The present author noted similarities in biochemistry and some symptoms between autism and CFS, and suspected that the same mechanism was involved, so that similar treatments should be effective [7]. A few people with CFS began trying Defeat Autism Now (DAN!) and Yasko treatments. • The present author presented the GD-MCB hypothesis for CFS at the IACFS conference in January, 2007 [8] • In late January, 2007, the present author suggested hypothesis testing using an orthomolecular simplified treatment approach for CFS (involving seven supplements) extracted from the complete treatment program of Amy Yasko, Ph. D. , N. D. , used primarily in autism [9]. • Starting on Feb. 19, 2007, some CFS patients began trying the simplified treatment approach. Number of supplements was decreased to five. Cost became less than $3. 00 per day. Initial results were quite striking. Use spread via on-line support groups, and soon a few clinicians started using it in their practices, some in response to reports from their patients. Currently there at least several hundred CFS patients being treated for methylation cycle block worldwide, and most are reporting continuing improvement. Structured clinical trials have not yet been performed.

Glutathione—What is it and what does it do? (10 -14) • • • A tripeptide, composed of glutamate, cysteine and glycine Found in all cells, blood, bile and epithelial lining fluid of the lung Synthesized by cells, particularly in the liver The most abundant thiol-containing substance in cells Has reduced and oxidized forms, GSH and GSSG Ratio of GSH to GSSG controls the redox potential in cells Serves as basis for the antioxidant system, quenching reactive oxygen species Conjugates several classes of toxins for removal from the body in Phase II detox, and quenches free radicals generated in Phase I detox in general Supports immune system, especially cell-mediated immunity Plays important role in synthesis of proteins that contain cysteine Participates in bile production Has many other roles

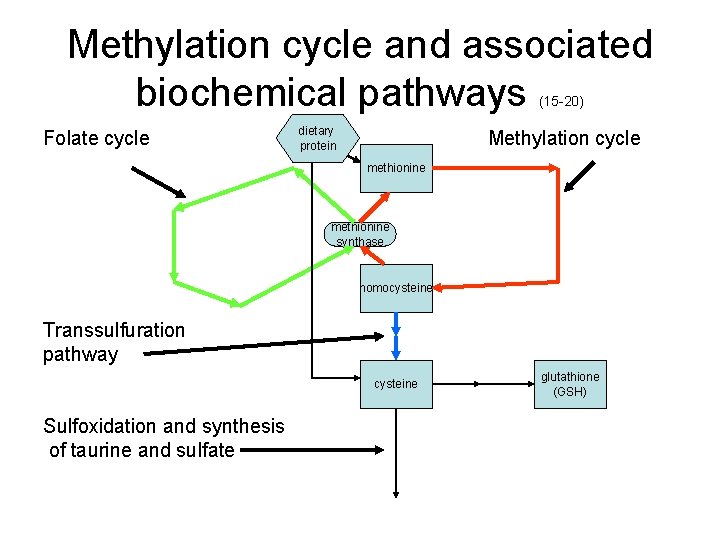
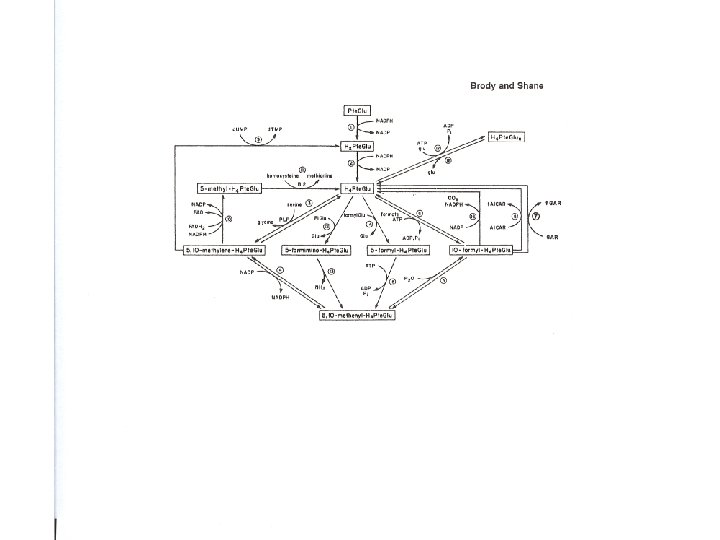
Methylation cycle and associated biochemical pathways (15 -20) dietary Folate cycle Methylation cycle protein methionine synthase homocysteine Transsulfuration pathway cysteine Sulfoxidation and synthesis of taurine and sulfate glutathione (GSH)

What does the methylation cycle do? (21) • Supplies methyl (CH 3) groups for a large number of biochemical reactions in the body. • Controls the overall sulfur metabolism, balancing the needs for methyl groups, for GSH to control oxidative stress, and for other sulfur metabolites, including cysteine, taurine and sulfate. • Coordinates the production of new DNA with the supply of methyl groups, which are used to methylate DNA, among many other roles.

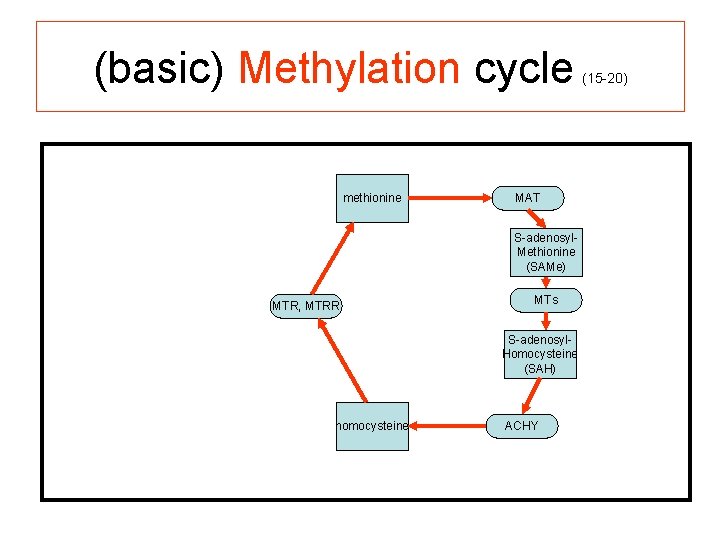
(basic) Methylation cycle methionine (15 -20) MAT S-adenosyl. Methionine (SAMe) MTR, MTRR MTs S-adenosyl. Homocysteine (SAH) homocysteine ACHY

(basic) Methylation cycle with BHMT pathway added (15 -20) [Note that BHMT is found only in liver and kidney cells(22). ] methionine MAT DMG MTR, MTRR MTs BHMT TMG (betaine) homocysteine S-adenosylmethionine S-adenosylhomocysteine AHCY

(complete) Methylation cycle dietary protein methionine NADPH MTR, MTRR MAT DMG BHMT TMG (betaine) B 12 homocysteine (15 -20) ATP S-adenosylmethionine PPi + Pi MTs methyl acceptor S-adenosylhomocysteine AHCY adenosine methylated product H 2 O

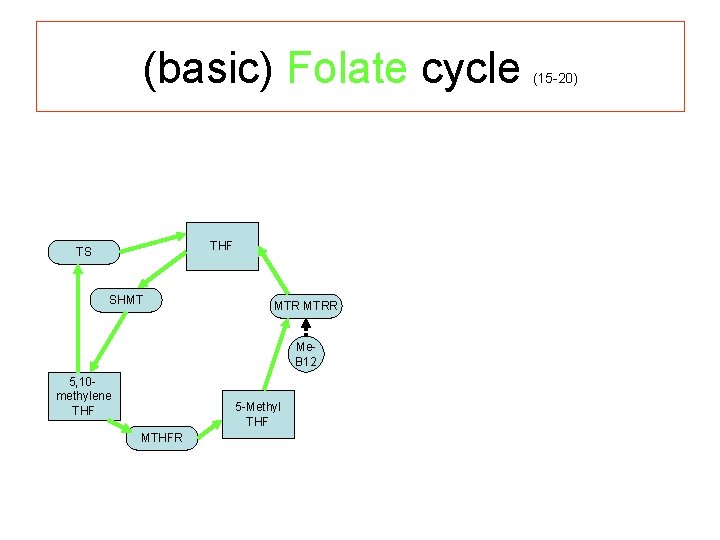
(basic) Folate cycle THF TS SHMT MTRR Me. B 12 5, 10 - methylene THF 5 -Methyl THF MTHFR (15 -20)

(more complete) Folate cycle thymidine synthesis (for DNA) d. UMP THF TS SHMT P 5 P (B 6) 5, 10 - methylene THF MTRR glycine Me. B 12 5 -Methyl THF MTHFR purine synthesis (for DNA/RNA) serine (15 -20)

(combined) Methylation and Folate cycles (15 -20) [Note that the methylation cycle and the folate cycle are present in all cells of the body (22). ] thymidine synthesis (for DNA) d. UMP dietary protein methionine THF TS NADPH SHMT P 5 P (B 6) 5, 10 - methylene THF MTRR glycine 5 -Methyl THF MTHFR purine synthesis (for DNA/RNA) serine MAT DMG MTs BHMT TMG (betaine) B 12 homocysteine S-adenosylmethionine S-adenosylhomocysteine AHCY adenosine ATP PPi + Pi methyl acceptor methylated product H 2 O

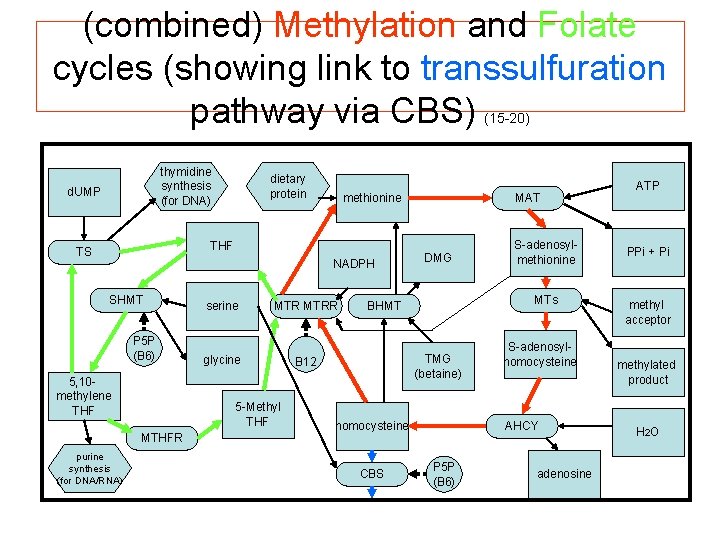
(combined) Methylation and Folate cycles (showing link to transsulfuration pathway via CBS) (15 -20) thymidine synthesis (for DNA) d. UMP dietary protein methionine THF TS NADPH SHMT P 5 P (B 6) 5, 10 - methylene THF MTRR glycine 5 -Methyl THF MTHFR purine synthesis (for DNA/RNA) serine MAT DMG MTs BHMT TMG (betaine) B 12 homocysteine CBS S-adenosylmethionine S-adenosylhomocysteine AHCY P 5 P (B 6) adenosine ATP PPi + Pi methyl acceptor methylated product H 2 O

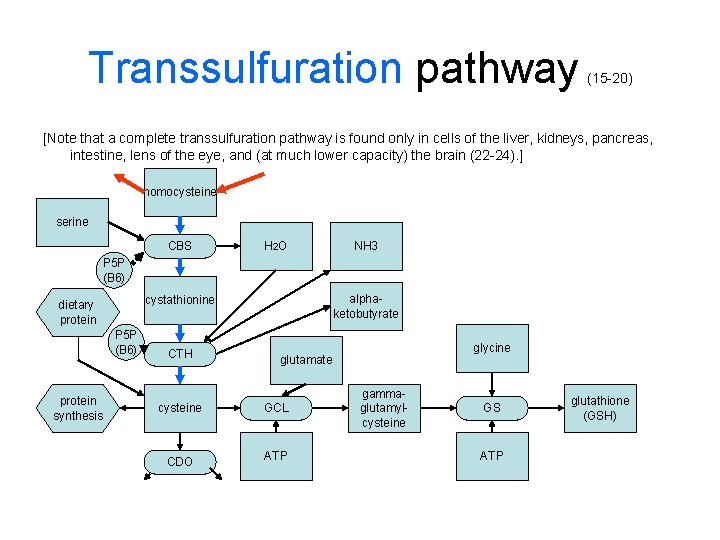
Transsulfuration pathway (15 -20) [Note that a complete transsulfuration pathway is found only in cells of the liver, kidneys, pancreas, intestine, lens of the eye, and (at much lower capacity) the brain (22 -24). ] homocysteine serine CBS H 2 O NH 3 P 5 P (B 6) protein synthesis alphaketobutyrate cystathionine dietary protein CTH cysteine CDO glycine glutamate GCL ATP gammaglutamylcysteine GS ATP glutathione (GSH)

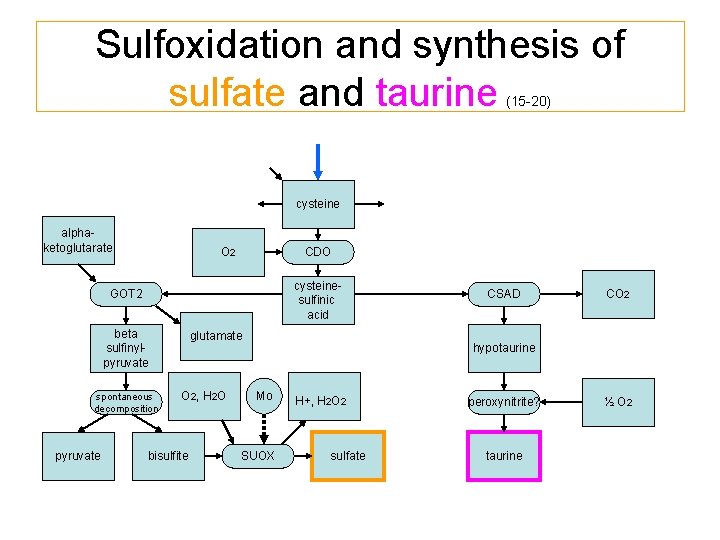
Sulfoxidation and synthesis of sulfate and taurine (15 -20) cysteine alphaketoglutarate O 2 CDO cysteinesulfinic acid GOT 2 beta sulfinylpyruvate spontaneous decomposition pyruvate CSAD glutamate O 2, H 2 O bisulfite CO 2 hypotaurine Mo SUOX peroxynitrite? sulfate taurine H+, H 2 O 2 ½ O 2

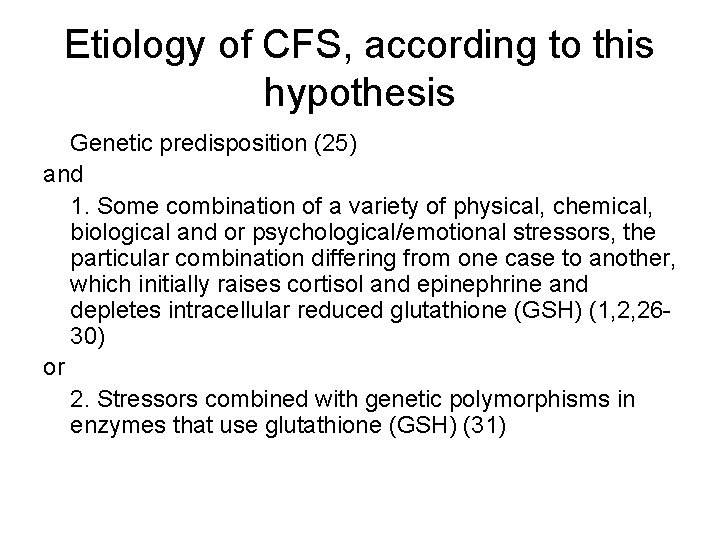
Etiology of CFS, according to this hypothesis Genetic predisposition (25) and 1. Some combination of a variety of physical, chemical, biological and or psychological/emotional stressors, the particular combination differing from one case to another, which initially raises cortisol and epinephrine and depletes intracellular reduced glutathione (GSH) (1, 2, 2630) or 2. Stressors combined with genetic polymorphisms in enzymes that use glutathione (GSH) (31)

Most common pathogenesis of CFS, according to this hypothesis 1. Stressors lower glutathione (GSH) (1, 2, 26 -30), which produces oxidative stress (27, 28, 30, 33 -44), allows toxins to accumulate (45 -48), and removes protection from B 12 (49). 2. Oxidative stress partially blocks methionine synthase (MTR) (50) and shifts cysteine toward cystine. 3. Accumulated toxins (probably especially mercury) react with much of the B 12 (49, 51, 52). 4. Partial block of methionine synthase (MTR) becomes chronic. 5. Cystathionine-gamma-lyase (CTH) converts cystine to hydrogen sulfide, which is then converted to thiosulfate (53). 6. Sulfur metabolites drain down to form thiosulfate, which is excreted, lowering methionine. 7. Intracellular cysteine levels become too low to restore glutathione levels to normal. 8. Resulting vicious circle becomes chronic.

Cystathionine gamma lyase (CTH) pathway (53, 54) diverts cysteine to thiosulfate under oxidative stress conditions (hypothesis) oxidative stress cysteine pyruvate cystine thiocysteine CTH cysteine non-enzymatic decomposition hydrogen sulfide bisulfite SUOX NH 4+ oxygen thiosulfate reductase? thiosulfate ? H 2 O GSH GSSG hydrogen sulfide Mo sulfate

A less common pathogenesis, according to this hypothesis 1. There are genetic polymorphisms in glutathione peroxidases (GPx) and/or glutathione transferases (GST’s), so that glutathione is not effectively used (31). 2. Stressors lead to same effects as above, even though glutathione levels do not drop, and may even be elevated (27). 3. Oxidative stress leads to partial block of methionine synthase (MTR) (50) and toxins build up (45 -48), reacting with B 12 (49, 51, 52). 4. Partial block becomes chronic.

Why do these pathogenetic processes take place in the people who develop CFS, but not in other people? • A major factor is likely to be differences in the combinations of inherited genetic polymorphisms. • There has not yet been a complete genome study of the polymorphisms that are more frequent in CFS than in the general population. • There is evidence from family and twin studies as well as limited polymorphism studies that there is a genetic component in the development of CFS (25).

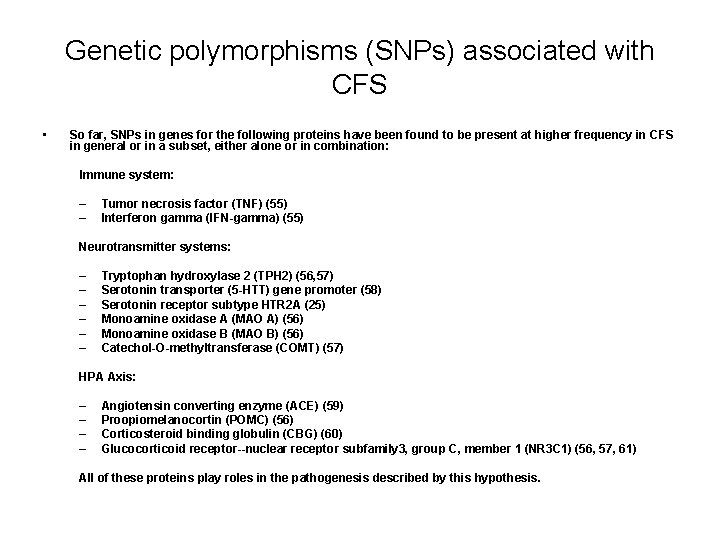
Genetic polymorphisms (SNPs) associated with CFS • So far, SNPs in genes for the following proteins have been found to be present at higher frequency in CFS in general or in a subset, either alone or in combination: Immune system: – – Tumor necrosis factor (TNF) (55) Interferon gamma (IFN-gamma) (55) Neurotransmitter systems: – – – Tryptophan hydroxylase 2 (TPH 2) (56, 57) Serotonin transporter (5 -HTT) gene promoter (58) Serotonin receptor subtype HTR 2 A (25) Monoamine oxidase A (MAO A) (56) Monoamine oxidase B (MAO B) (56) Catechol-O-methyltransferase (COMT) (57) HPA Axis: – – Angiotensin converting enzyme (ACE) (59) Proopiomelanocortin (POMC) (56) Corticosteroid binding globulin (CBG) (60) Glucocorticoid receptor--nuclear receptor subfamily 3, group C, member 1 (NR 3 C 1) (56, 57, 61) All of these proteins play roles in the pathogenesis described by this hypothesis.

Accounting for observed biochemical abnormalities, pathophysiology and symptoms with this hypothesis • “Gedanken experiment” approach: examine the normal functions of glutathione and methylation, and consider what might be expected to occur if these functions were not carried out. • It is found that this “gedanken experiment” approach reproduces many observed features of CFS in detail. • Features that were not discovered and explained by this approach can be traced back to these same causes by starting with the features and considering what might cause them, i. e. reasoning in the opposite direction. • The result of this bidirectional thought process is that essentially all the observed features of CFS can be specifically accounted for by this hypothesis.

What are some things that might be expected if glutathione were depleted, and are they observed in CFS? • • Oxidative stress—observed (27, 28, 30, 33 -44). Mitochondrial dysfunction and low ATP output, leading, for examples, to physical fatigue in skeletal muscles—observed; and diastolic dysfunction in the heart, leading to low cardiac output—observed (62) Buildup of toxins, including heavy metals—observed (5, 46). Immune response shift to Th 2—observed (63). Inability of T cells to proliferate in response to mitogens—observed (64). Reactivation of herpes family viral infections—observed (65). Thyroid problems—observed (66). Low secretion and dysregulation of certain cysteine-containing secretory proteins, including ACTH, antidiuretic hormone, and perforin (67). Low ACTH leads to blunting of the HPA axis—observed (68), low antidiuretic hormone leads to high daily urine volumes and constant thirst—observed (69), and low perforin leads to low cytotoxic activity of the natural killer cells and the CD 8 (“killer”) T cells—observed (70).

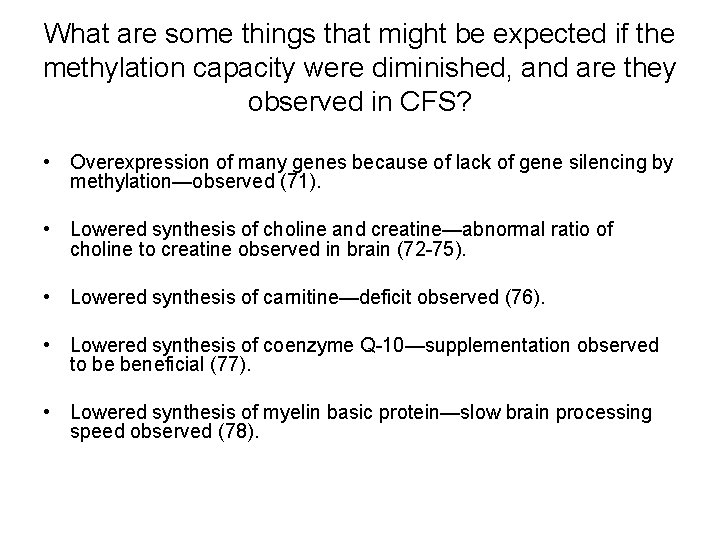
What are some things that might be expected if the methylation capacity were diminished, and are they observed in CFS? • Overexpression of many genes because of lack of gene silencing by methylation—observed (71). • Lowered synthesis of choline and creatine—abnormal ratio of choline to creatine observed in brain (72 -75). • Lowered synthesis of carnitine—deficit observed (76). • Lowered synthesis of coenzyme Q-10—supplementation observed to be beneficial (77). • Lowered synthesis of myelin basic protein—slow brain processing speed observed (78).

How does this hypothesis account for the higher prevalence of CFS in women than in men? • During their potentially reproductive years, estrogens are produced in larger amounts in women, and must be metabolized. • Some people (both men and women) inherit polymorphisms in the genes that code for some of the detox enzymes involved in the metabolism of the estrogens (CYP 1 B 1, COMT and GST enzymes). • In women, these polymorphisms can lead to redox cycling when metabolizing estrogens. This adds an additional bias toward depletion of glutathione and development of oxidative stress. • Oxidative stress initiates the pathogenesis of CFS. • (For more details, see 2007 IACFS poster paper: http: //phoenixcfs. org/Gender. CFSKonynenburg. htm )

Lab testing • The methylation panel (offered by Vitamin Diagnostics, Inc. , and the European Laboratory of Nutrients) is the most definitive for detecting methylation cycle block and glutathione depletion. • Urine testing for methylmalonic acid and formiminoglutamic acid (“figlu”) are also very helpful. When these are elevated, they indicate low adenosylcobalamin and low tetrahydrofolate, respectively. When both methylamalonic acid and figlu are elevated, it is very likely that methionine synthase is partially blocked.

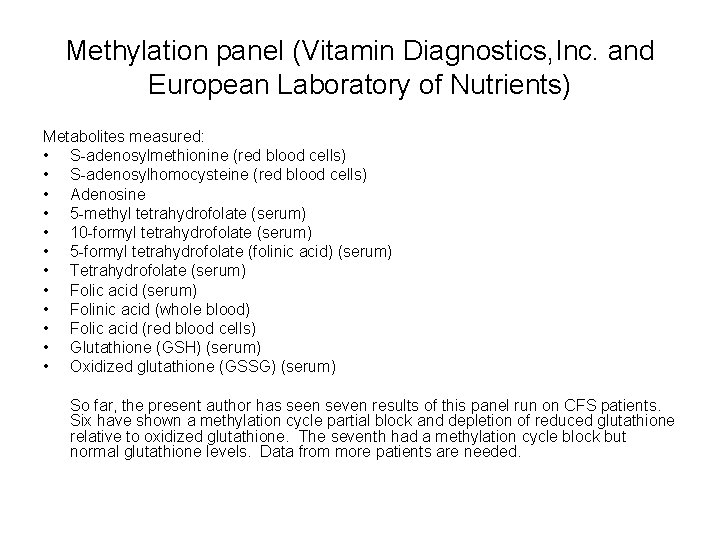
Methylation panel (Vitamin Diagnostics, Inc. and European Laboratory of Nutrients) Metabolites measured: • S-adenosylmethionine (red blood cells) • S-adenosylhomocysteine (red blood cells) • Adenosine • 5 -methyl tetrahydrofolate (serum) • 10 -formyl tetrahydrofolate (serum) • 5 -formyl tetrahydrofolate (folinic acid) (serum) • Tetrahydrofolate (serum) • Folic acid (serum) • Folinic acid (whole blood) • Folic acid (red blood cells) • Glutathione (GSH) (serum) • Oxidized glutathione (GSSG) (serum) So far, the present author has seen seven results of this panel run on CFS patients. Six have shown a methylation cycle partial block and depletion of reduced glutathione relative to oxidized glutathione. The seventh had a methylation cycle block but normal glutathione levels. Data from more patients are needed.

Testing the GD-MCB hypothesis by using treatment based on it • The main goal of such treatment would be to raise the activity of methionine synthase. • This appears to be best done by the simultaneous application of bioactive forms of vitamin B 12 and bioactive forms of folate, since both are required by methionine synthase (MTR), and often both are deficient. • Some support for the BHMT pathway would likely also be helpful, since this promotes synthesis of SAMe, which supports methionine synthase reductase (MTRR). • General nutritional support would likely be helpful as well, since many CFS patients have nutritional deficiencies.

“Simplified Treatment Approach” • Derived from part of “step 2” of the full methylation cycle block treatment program developed by Amy Yasko, Ph. D. , N. D. and used primarily in autism (9). • Consists of five nutritional supplements, taken daily: 1. Hydroxocobalamin (2, 000 micrograms, sublingual) 2. 5 -Methyl tetrahydrofolate (200 mcg) 3. Combination of [folic acid, 5 -methyl tetrahydrofolate, and folinic acid] (200 mcg), cyanocobalamin (125 mcg), calcium (22. 5 mg), phosphorus (17. 25 mg), and intrinsic factor (5 mg) 4. A multivitamin, multimineral supplement including antioxidants, trimethylglycine, nucleotides, supplements to support the sulfur metabolism, a high ratio of magnesium to calcium, and no iron or copper (up to two tablets) 5. Phosphatidyl serine complex (one softgel) Note: Even though this treatment consists only of nutritional supplements, patients who are on it need to be under the care of a physician, in order that any individual health issues that may arise may be properly dealt with.

Composition of multi-vitamin, multi-mineral supplement used in “simplified treatment approach” • Serving Size: 6 Tablets (note that up to 2 tablets per day are used in the treatment) • Amount per serving: Vitamin A (as palmitate)5000 IU, Vitamin C (ascorbic acid)500 mg, Vitamin D (as cholecaliciferol)400 IU, Vitamin E (as d-alpha tocopheryl succinate)400 IU, Vitamin K (as phytonadione)40 mcg, Vitamin B-1 (as benfotiamine)25 mg, Vitamin B-2 (as riboflavin)12. 5 mg, Niacin (as niacinamide)37. 5 mg, Vitamin B-6 (as pyridoxal-5 -phosphate)12. 5 mg, Folic Acid 100 mcg, Vitamin B-12 (cyanocobalamin B 12)250 mcg, Biotin 150 mcg, Pantothenic Acid (as d-calcium pantothenate)50 mg, Calcium (as calcium d-glucarate)25 mg, Magnesium (as citrate, oxide)100 mg, Zinc (as monomethionine)5 mg, Selenium (as L-selenomethionine)100 mcg, Manganese (as arginate)1 mg, Chromium (as polynicotinate)100 mcg, Molybdenum (as amino acid chelate)75 mcg, Potassium (as citrate)5 mg, Broccoli florets powder 160 mg, Citrus bioflavonoids 50 mg, Choline (as bitartrate)25 mg, Inositol 25 mg, PABA (para-amino benzoic acid)5 mg, Garlic (Allium sativum) bulb powder 200 mg, L-methionine 150 mg, Milk thistle (Silybum marianum) seed extract 100 mg, N-acetyl-cysteine 75 mg, Pine (Pinus maritimus) bark extract 25 mg, Taurine 250 mg, Turmeric (Curcuma longa) root extract 50 mg, Intrinsic Factor 5 mg, Trimethylglycine (TMG)50 mg, Free Form Nucleotide Complex 100 mg, Boron 1 mg, L-Carnitine (Tartrate)100 mg. • (Ref. : http: //www. holisticheal. com)

Results of treatment • Informal reports from clinicians • Informal reports from patients Beneficial changes Detox and die-off symptoms Serious adverse effects reported by a few patients.

Informal reports from clinicians (conveyed with their permission) • David Bell, M. D. (Lyndonville, NY): “I have a good treatment response in roughly 50% of my long-term patients who have not responded particularly well to standard symptom-based therapies. I am very encouraged…” • Karen Vrchota, M. D. (Winona, MN): “ 78 out of 109 patients [72%] have marked improvement. ” Patients are “slowly improving week to week and month to month. Those started in July 2007 have not peaked yet; that is, they are still improving. ” • Neil Nathan, M. D. (Springfield, MO): “I’ve got about 75 patients on the protocol now, and have results from about 60. Roughly, 70% report noticeable improvement, and 15 to 20% report marked improvement. 30 to 40 % report reactions in one form or another. Most of these are very mild. ” “It's clear that it does work. We now have to define how to use it optimally. ”

Informal reports from clinicians (conveyed with their permission) (continued) • Derek Enlander, M. D. (NYC): Using his own protocol, which includes methylation cycle treatment (but does not include 5 -methyl THF), Dr. E. reports that he has 112 patients under treatment, and that 65 to 70% of them show improvement. • Sarah Myhill, MB BS (Wales): Dr. M. has 10 -12 CFS patients on her methylation supplement package, but does not yet have feedback from all of them. However, she reports that “There is no doubt that for some this is a very worthwhile intervention. ” • Jacob Teitelbaum, M. D. (Hawaii): “Some doctors in the Fibromyalgia and Fatigue Centers of America have started using the protocol. I am excited about its potential and am awaiting feedback. ”

Beneficial changes reported by various patients • • • Improvement in sleep (though a few have reported increased difficulty in sleeping initially). Ending of the need for and intolerance of continued thyroid hormone supplementation. Termination of excessive urination and night-time urination. Restoration of normal body temperature from lower values. Restoration of normal blood pressure from lower values. Initiation of attack by immune system on longstanding infections. Increased energy and ability to carry on higher levels of activity without postexertional fatigue or malaise. Termination of “crashing. ” Lifting of brain fog, increase in cognitive ability, return of memory. Relief from hypoglycemia symptoms. Improvement in alcohol tolerance. Decrease in pain (though some have experienced increases in pain temporarily, as well as increased headaches, presumably as a result of detoxing).

Beneficial changes reported by various patients (continued) • • • Notice of and remarking by friends and therapists on improvements in the patient’s condition. Necessity to adjust relationship with spouse, because not as much caregiving is needed. Need to work out more balanced responsibilities in relationship in view of improved health and improved desire and ability to be assertive. Return of ability to read and retain what has been read. Return of ability to take showers standing up. Return of ability to sit up for long times. Return of ability to drive for long distances. Improved tolerance for heat. Feeling unusually calm. Feeling "more normal and part of the world. " Ability to stop steroid hormone support without experiencing problems from doing it. Lowered sensation of being under stress. Loss of excess weight.

Detox and die-off related symptoms reported by various patients • • • • Headaches, “heavy head, ” “heavy-feeling headaches. ” Alternated periods of mental “fuzziness” and greater mental clarity. Feeling “muggy-headed” or “blah” or sick in the morning. Transient malaise, flu-like symptoms. Transiently increased fatigue, waxing and waning fatigue, feeling more tired and sluggish, weakness. Dizziness. Irritability. Sensation of “brain firing: bing, bong, ” “brain moving very fast. ” Depression, feeling overwhelmed, strong emotions. Greater need for “healing naps. ” Swollen or painful lymph nodes. Mild fevers. Runny nose, low grade “sniffles, ” sneezing, coughing.

Detox and die-off related symptoms reported by various patients (continued) • • • • Sore throat. Rashes. Itching. Increased perspiration, unusual smelling perspiration. “Metallic” taste in mouth. Transient nausea, “sick to stomach. ” Abdominal cramping/pain. Increased bowel movements. Diarrhea, loose stools, urgency. Unusual color of stools, e. g. green. Temporarily increased urination. Transiently increased thirst. Clear urine.

Serious adverse effects reported by a few patients • • • Exacerbation of comorbid autoimmune disease. Exacerbation of comorbid autonomous multinodular goiter. Cessation of peristalsis for two weeks. Persisting low fever of unknown origin. Flare-up of Lyme disease that had been controlled with antibiotics. Conclusion from this experience: Even though this treatment consists only of food supplements, structured clinical trials are needed to determine the efficacy of this treatment quantitatively and to learn how it may be safely applied.

Some questions that remain to be answered 1. For which PWCs would this be an appropriate treatment approach? 2. For what fraction of the entire PWC population will this treatment approach be beneficial? 3. How can PWCs who are likely to experience adverse effects from this treatment approach be identified beforehand, so that these effects can be avoided? 4. Are there PWCs who are too debilitated to be able to tolerate the detoxing and die-off processes that result from this treatment approach, and if so, would the full Yasko treatment approach be suitable for them? 5. Will the simplified treatment approach produce continued improvement over time for those who are finding it beneficial, and will they be cured? 6. Will the simplified treatment approach be effective in cases of "pure fibromyalgia" as it appears to be in many cases of CFS? 7. How can this treatment approach be further improved?

Planned clinical study • • Objective: Determine effectiveness of a treatment to lift the methylation cycle block 100 patients, satisfying diagnostic criteria for both CFS and fibromyalgia, in one practice (Neil Nathan, M. D. , Springfield, MO) Informed consent Lab testing (2 X): Methylation panel, characterization of certain polymorphisms, thyroid panel including autoantibodies Questionnaires to collect pertinent data and evaluate symptoms (3 X) Treatment—”simplified treatment approach” (five supplements daily) Patient logs Treatment duration—six months This study will not be randomized, doubly blinded, or placebo controlled, but hopefully it will demonstrate that the treatment is worthy of a more controlled study.

The Bottom Line • A comprehensive biochemical hypothesis has been developed to explain the etiology, pathogenesis, pathophysiology and symptomatology of chronic fatigue syndrome (CFS). • The key biochemical features of this hypothesis are a chronic partial block of the methylation cycle at methionine synthase and a chronic depletion of glutathione. • This hypothesis explains the observed genetic predisposition, observed biochemical abnormalities, and many seemingly disparate symptoms of CFS as reported in the peer-reviewed literature and as observed clinically. • Lab testing is available to test this hypothesis and to determine whether it applies to a particular patient. So far it appears to apply to most CFS patients. • This hypothesis is also being tested by using orthomolecular treatment including biochemically active forms of vitamin B 12 and folate. It is currently being applied to at least several hundred patients by at least ten clinicians and is producing significant benefits in most patients. A preliminary clinical study of this treatment is planned.

Additional reading • • Van Konynenburg, R. A. , “Is Glutathione Depletion an Important Part of the Pathogenesis of Chronic Fatigue Syndrome? ” poster paper, AACFS 7 th Intl. Conf. , Madison, WI, October 8 -10, 2004 http: //phoenix-cfs. org/Glu. AACFS 04. htm Van Konynenburg, R. A. , “Glutathione Depletion—Methylation Cycle Block, A Hypothesis for the Pathogenesis of Chronic Fatigue Syndrome, ” poster paper, 8 th Intl. IACFS Conf. on CFS, Fibromyalgia, and Other Related Illnesses, Fort Lauderdale, FL, January 10 -14, 2007 http: //phoenix-cfs. org/GSHMethylation. Van. Konynenburg. htm • Van Konynenburg, R. A. , “Why is the Prevalence of Chronic Fatigue Syndrome Higher in Women than in Men? ” poster paper, 8 th Intl. IACFS Conf. on CFS, Fibromyalgia, and Other Related Illnesses, Fort Lauderdale, FL, January 10 -14, 2007 http: //www. phoenix-cfs. org/Res. Gender. CFSKonynenburg. htm • Van Konynenburg, R. A. , “Simplified Treatment Approach Based on the Glutathione Depletion. Methylation Cycle Block Pathogenesis Hypothesis for Chronic Fatigue Syndrome (CFS), ” article, July 18, 2007 http: //phoenix-cfs. org/GSHMethyl. Depl. Theory. July 07. htm and http: //phoenix-cfs. org/GSHMethyl. Trt. Plan. July 07. htm

References 1. Cheney, P. R. , Evidence of glutathione deficiency in chronic fatigue syndrome, American Biologics 11 th International Symposium (1999), Vienna, Austria, Tape no. 07 -199, available from Professional Audio Recording, P. O. Box 7455, La. Verne, CA 91750 (phone 1 -800 -227 -4473). 2. Cheney, P. R. , Chronic fatigue syndrome, lecture presented to the CFIDS Support Group of Dallas-Fort Worth, Euless, TX, on May 15, 1999. Video tape available from Carol Sieverling, 513 Janann St. , Euless, TX 76039. 3. Enlander, D. , personal communication, 2007. 4. Salvato, P. , CFIDS patients improve with glutathione injections, CFIDS Chronicle (Jan/Feb 1998). 5. Van Konynenburg, R. A. , “Is Glutathione Depletion an Important Part of the Pathogenesis of Chronic Fatigue Syndrome? ” poster paper, AACFS 7 th Intl. Conf. , Madison, WI, October 8 -10, 200 http: //phoenix-cfs. org/Glu. AACFS 04. htm 6. James, S. J. , Cutler, P. , Melnyk, S. , Jernigan, S. , Janak, L. , Gaylor, D. W. , and Neubrander, J. A. , Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism, Clin. Nutrit. 2004; 80: 1611 -1617. 7. Van Konynenburg, R. A. , Chronic fatigue syndrome and autism, Townsend Letter for Doctors and Patients, October 2006, paper available at http: //www. findarticles. com/p/articles/mi_m. OISW/is_279/ai_n 16865315/print 8. Van Konynenburg, R. A. , “Glutathione Depletion—Methylation Cycle Block, A Hypothesis for the Pathogenesis of Chronic Fatigue Syndrome, ” poster paper, 8 th Intl. IACFS Conf. on CFS, Fibromyalgia, and Other Related Illnesses, Fort Lauderdale, FL, January 10 -14, 2007 http: //phoenix-cfs. org/GSHMethylation. Van. Konynenburg. htm

References (continued) 9. Yasko, A. and Gordon, G. , The Puzzle of Autism: Putting it All Together, Matrix, Payson, AZ (2006). 10. Wu, G. , Fang, Y. -Z. , Yang, S. , Lupton, J. R. , and Turner, N. D. , Glutathione metabolism and its implications for health, J. Nutr. (2004) 134: 489 -492. 11. Dickinson, D. A. , Moellering, D. R. , Iles, K. E. , Patel, R. P. , Levonen, A. -L. , Wigley, A. , Darley-Usmar, V. M. , and Forman, H. J. , Cytoprotection against oxidative stress and the regulation of glutathione synthesis, Biol. Chem. (2003) 384: 527 -537. 12. Lu, S. C. , Regulation of hepatic glutathione synthesis: current concepts and controversies, FASEB J. (1999) 13: 1169 -1183. 13. Kidd, P. M. , Glutathione: systemic protectant against oxidative and free radical damage, Alt. Med. Rev. (1997) 1: 155 -176. 14. Lomaestro, B. M. , and Malone, M. , Glutathione in health and disease: pharmacotherapeutic issues, Ann. Pharmacother. (1995) 29: 1263 -1273. 15. Devlin, T. M. , editor, Textbook of Biochemistry With Clinical Correlations, Sixth Edition, Wiley-Liss, Hoboken, NJ (2006). 16. Finkelstein, J. D. , Inborn errors of sulfur-containing amino acid metabolism, J. Nutr. 136: 1750 S-1754 S (2006). 17. Stipanuk, M. H. , Londono, M. , Lee, J-I. , Hu, M. , and Yu, A. F. , Enzymes and metabolites of cysteine metabolism in nonhepatic tissues of rats show little response to changes in dietary protein or sulfur amino acid levels, J. Nutr. 132: 3369 -3378 (2002).

References (continued) 18. Pangborn, J. , and Baker, S. M. , Autism: effective biomedical treatments, Autism Research Institute, San Diego, CA (2005). 19. Reed, M. C. , Nijhout, H. F. , Neuhouser, M. L. , Gregory, J. F. III, Shane, B. , James, S. J. , Boynton, A. , and Ulrich, C. M. , A mathematical model gives insights into nutritional and genetic aspects of folate-mediated one-carbon metabolism, J. Nutr. 136: 2653 -2661 (2006). 20. Fontana, M. , Amendola, D. , Orsini, E. , Boffi, A. and Pecci, L. , Oxidation of hypotaurine and cysteine sulphinic acid by peroxynitrite, Bichem. J. 389: 233 -240 (2005). 21. Deth, R. , Muratore, C. , Benzecry, J. , Power-Charnitsky, V. A. , and Waly, M. , How environmental and genetic factors combine to cause autism: a redox/methylation hypothesis, Neurotoxicology 29 (1): 190 -201 (2008). 22. Finkelstein, J. D. , Methionine metabolism in liver diseases, Am J Clin Nutr 77: 1094 -5 (2003). 23. Persa, C. , Pierce, A. , Ma, Z. , Kabil, O. , and Lou, M. F. , The presence of a transsulfuration pathway in the lens: a new oxidative stress defense system, Experimental Eye Research 79: 875 -886 (2004). 24. Vitvitsky, V. , Thomas, M. , Ghorpade, A. , Gendelman, H. E. , and Banerjee, R. , A functional transsulfuration pathway in the brain links to glutathione homeostasis, J Biol Chem 281(47): 35785 -35793 (2006). 25. Smith, A. K. , Dimulescu, I. , Falkenberg, V. R. , Narasimhan, S. , Heim, C. , Vernon, S. D. , and Rajeevan, M. S. , Genetic evaluation of the serotonergic system in chronic fatigue syndrome, Psychoneuroendocrinology 33(2): 188 -197 (2008). 26. Droge, W. and Holm, E. , Role of cysteine and glutathione in HIV infection and other diseases associated with muscle wasting and immunological dysfunction, FASEB J (1997), 11: 1077 -1089.

References (continued) 27. Richards, R. S. , Roberts, T. K. , Dunstan, R. H. , Mc. Gregor, N. R. , and Butt, H. L. , Free radicals in chronic fatigue syndrome: cause or effect? , Redox Report (2000) 5 (2/3): 146 -147. 28. Manuel y Keenoy, B. , Moorkens, G. , Vertommen, J. , Noe, M. , Neve, J. , and De Leeuw, I. , Magnesium status and parameters of the oxidant-antioxidant balance in patients with chronic fatigue: effects of supplementation with magnesium, J Amer Coll Nutrition (2000), 19(3): 374 -382. 29. Kurup, R. K. , and Kurup, P. A. , Hypothalamic digoxin, cerebral chemical dominance and myalgic encephalomyelitis, Intern J Neurosci (2003), 113: 683 -701. 30. Kennedy, G. , Spence, V. A. , Mc. Laren, M. , Hill, A. , Underwood, C. , and Belch, J. J. , Oxidative stress levels are raised in chronic fatigue syndrome and are associated with clinical symptoms, Free Radic Biol Med (2005), 39(5): 584 -589. 31. Hashimoto, T. , Hashimoto, K. , Miyatake, R. , Matsuzawa, D. , Sekine, Y. , Inada, T. , Ozaki, N. , Iwata, N. , Harano, M. , Komiyama, T. , Yamada, M. , Sora, I. , Ujike, H. , and Iyo, M. , Association study between polymorphisms in glutathione-related genes a methamphetamine use disorder in a Japanese population Am J Med Genet B Neuropsychiatr Genet. 2008 Jan 9 [Epub ahead of print]. 32. Babior, B. M. , and Bunn, H. F. , Chapter 92: Megaloblastic anemias, pp. 601 -607 in Harrison’s Principles of Internal Medicine, 16 th edition, D. L. Kasper et al. , eds. , Mc. Graw-Hill, New York (2005). 33. Ali, M. , Ascorbic acid reverses abnormal erythrocyte morphology in chronic fatigue syndrome, abstract, Am. J. Clin. Pathol. (1990); 94: 515. 34. Ali, M. , Hypothesis: chronic fatigue is a state of accelerated oxidative molecular injury, J. Advancement in Med. (1993); 6 (2): 83 -96.

References (continued) 35. Richards, R. S. , Roberts, T. K. , Mc. Gregor, N. R. , Dunstan, R. H. , and Butt, H. L. , Blood parameters indicative of oxidative stress are associated with symptom expression in chronic fatigue syndrome, Redox Report (2000); 5 (1): 35 -41. 36. Fulle, S. , Mecocci, P. , Fano, G. , Vecchiet, I. , Vecchini, A. , Racciotti, D. , Cherubini, A. , Pizzigallo, E. , Vecchiet, L. , Senin, U. , and Beal, M. F. , Specific oxidative alterations in vastus lateralis muscle of patients with the diagnosis of chronic fatigue syndrome, Free Radical Biol. and Med. (2000); 29 (12): 1252 -1259. 37. Manuel y Keenoy, B. , Moorkens, G. , Vertommen, J. , and De Leeuw, I. , Antioxidant status and lipoprotein peroxidation in chronic fatigue syndrome, Life Sciences (2001); 68: 2037 -2049. 38. Vecchiet, J. , Cipollone, F. , Falasca, K. , Mezzetti, A. , Pizzigallo, E. , Bucciarelli, T. , De Laurentis, S. , Affaitati, G. , De Cesare, D. , Giamberardino, M. A. , Relationship between musculoskeletal symptoms and blood markers of oxidative stress in patients with chronic fatigue syndrome, Neuroscience Letts. (2003); 335: 151 -154. 39. Smirnova, I. V. , and Pall, M. L. , Elevated levels of protein carbonyls in sera of chronic fatigue syndrome patients, Molecular and Cellular Biochem. (2003); 248: 93 -95. 40. Jammes, Y. , Steinberg, J. G. , Mambrini, O. , Bregeon, F. , and Delliaux, S. , Chronic fatigue syndrome: assessment of increased oxidative stress and altered muscle excitability in response to incremental exercise, J. Intern. Med. (2005); 257 (3): 299 -310. 41. Maes, M. , Mihaylova, I. and Leunis, J. C. , Chronic fatigue syndrome is accompanied by an Ig. M-related immune response directed against neopitopes formed by oxidative or nitrosative damage to lipids and proteins, Neuro Endocrinol. Lett. (2006); 27 (5): 615 -621.

References (continued) 42. Richards, R. S. , Wang, L. , and Jelinek, H. , Erythrocyte oxidative damage in chronic fatigue syndrome, Arch. Med. Res. (2007); 38 (1): 94 -98. 43. Maes, M. , Mihaylova, I. , and Bosmans, E. , Not in the mind of neurasthenic lazybones but in the cell nucleus: patients with chonic fatigue syndrome have increased production of nuclear factor kappa beta, Neuro Endocrinol. Lett. 28(4), 456 -462 (2007). 44. Spence, V. A. , Kennedy, G. , Belch, J. J. , Hill, A. , and Khan, F. , Low grade inflammation and arterial wave reflection in patients with chronic fatigue syndrome, Clin. Sci. (Lond. ) (2007) Nov. 21 [Epub ahead of print]. 45. Dunstan, R. H. , Donohoe, M. , Taylor, W. , Roberts, T. K. , Murdoch, R. N. , Watkins, J. A. , and Mc. Gregor, N. R. , A preliminary investigation of chlorinated hydrocarbons and chronic fatigue syndrome, Med J Aust 1995 Sep 18; 163(6): 294 -7. 46. Racciatti, D. , Vecchiet, J. , Ceccomancini, A. , Ricci, F. , and Pizzigallo, E. , Chronic fatigue syndrome following a toxic exposure, Sci Total Environ. 2001 Apr 10; 270(1 -3): 27 -31. 47. Nogue, S. , Fernandez-Sola, J. , Rovira, E. , Montori, E. , Fernandez-Huerta, J. M. and Munne, P. , Multiple chemical sensitivity: study of 52 cases, Med Clin (Barc). 2007 Jun 16; 129(3): 96 -8; quiz 99. 48. Lindh, U. , Hudecek, R. , Danersund, A. , Eriksson, S. , Lindvall, A. , Removal of dental amalgams and other metal alloys supported by antioxidant therapy alleviates symptoms and improves quality of life in patients with amalgam-associated ill health, Neuro Endocrinol Lett. 2002 Oct-Dec; 23(5 -6): 459 -82.

References (continued) 49. Watson, W. P. , Munter, T. and Golding, B. T. , A new role for glutathione: protection of vitamin B 12 from depletion by xenobiotics, Chem. Res. Toxicol. 17: 1562 -1567 (2004). 50. Deth, R. , Muratore, C. , Benzecry, J. , Power-Charnitsky, V. A. , and Waly, M. , How environmental and genetic factors combine to cause autism: A redox/methylation hypothesis, Neurotoxicology. 2008 Jan; 29(1): 190 201. 51. De. Simone R. E. , Penley, M. W. , Charbonneau, L, Smith, S. G. , Wood, J. M. , Hill, H. A. , Pratt, J. M. , Ridsdale, S. , and Williams, R. J. , The kinetics and mechanism of cobalamin-dependent methyl and ethyl transfer to mercuric ion, Biochim Biophys Acta 1973 May 28; 304(3): 851 -63. 52. Waly, M. , Olteanu, H. , Banerjee, R. , Choi, S-W. , Mason, J. B. , Parker, B. S. , Sukumar, S. , Shim, S. , Sharma, A. , Benzecry, J. M. , Power-Charnitsky, V-A. , and Deth, R. C. , Activation of methionine synthase by insulin like growth factor-1 and dopamine: a target for neurodevelopmental toxins and thimerosal, Molec Psychiatry. 2004, Apr; 9(4): 358 -70. 53. Stipanuk, M. H. , Sulfur amino acid metabolism: pathways for production and removal of homocysteine and cysteine, Annu Rev Nutr 24: 539 -577 (2004). 54. Stipanuk, M. H. , and Beck, P. W. , Characterization of the enzymatic capacity for cysteine desulfhydration in liver and kidney of rat, Biochem J 206: 267 -277 (1982). 55. Carlo-Stella, N. , Badulli, C. , De Silvestri, A. , Bazzichi, L. , Martinetti, M. , Lorusso, L. , Bombardieri, S. , Salvaneschi, L. , and Cuccia, M. , A first study of cytokine genomic polymorphisms in CFS: positive association of TNF-857 and IFNgamma 874 rare alleles, Clin. Exp. Rheumatol. (2006); 24 (2): 179 -182.

References (continued) 56. Smith, A. K. , White, P. D. , Aslakson, E. , Vollmer-Conna, U. , and Rajeevan, M. S. , Polymorphisms in genes regulating the HPA axis associated with empirically delineated classes of unexplained chronic fatigue, Pharmacogenomics (2006); 7 (3): 387 -394. 57. Goertzel, B. N. , Pennachin, C. , Coelho, L. de S. , et al. , Combinations of single nucleotide polymorphisms in neuroendocrine effector and receptor genes predict chronic fatigue syndrome, Pharmacogenomics (2006); 7 (3): 475 -483. 58. Narita, M. , Nishigami, N. , Narita, N. , Yamaguti, K. , Okado, N. , Watanabe, Y. , and Kuratsune, H. , Association between serotonin transporter gene polymorphism and chronic fatigue syndrome, Biochem. Biophys. Res. Commun. (2003); 311 (2): 264 -266. 59. Vladutiu, G. D. , and Natelson, B. H. , Association of medically unexplained fatigue with ACE insertion/deletion polymorphism in Gulf War veterans, Muscle Nerve 30(1): 38 -43 (2004). 60. Torpy, D. J. , Bachmann, A. W. , Gartside, M. , Grice, J. E. , Harris, J. M. , Clifton, P. , Easteal, S. , Jackson, R. V. , Whitworth, J. A. , Association between chronic fatigue syndrome and the corticosteroid-binding globulin gene ALA SER 224 polymorphism, Endocr. Res. (2004); 30 (3): 417 -429. 61. Rajaveen, M. S. , Smith, A. K. , Dimulescu, I. , Unger, E. R. , Vernon, S. D. , Heim, C. , and Reeves, W. C. , Glucocorticoid receptor polymorphisms and haplotypes associated with chronic fatigue syndrome, Genes Brain Behav. (2007), 6: 167 -176, 62. Peckerman, A. , La. Manca, J. J. , Dahl, K. A. , Chemitiganti, R. , Qureishi, B. , and Natelson, B. H. , Abnormal impedance cardiography predicts symptom severity in chronic fatigue syndrome, Am. J. Med. Sci. (2003); 326(2): 55 -60.

References (continued) 63. Skowera, A. , Cleare, A. , Blair, D. , Bevis, L. , Wessely, S. C. , and Peakman, M. , High levels of type 2 cytokine producing cells in chronic fatigue syndrome, Clin. Exp. Immunol. (2004) 135: 294 -302. 64. Maher, K. J. , Klimas, N. G. , and Fletcher, M. A. , Immunology, chapter 7 in Handbook of Chronic Fatigue Syndrome (2003), L. A. Jason, P. A. Fennell, and R. R. Taylor, eds. , Wiley, Hoboken, NJ, pp. 124 -151. 65. Komaroff, A. L. , and Buchwald, D. S. , Chronic fatigue syndrome: an update, Annual Reviews of Medicine (1998) 49: 1 -13. 66. Wikland, B. , Lowhagen, T. , and Sandberg, P. O. . Fine-needle aspiration cytology of the thyroid in chronic fatigue, Lancet (2001); 357 (9260): 956 -957. 67. Chakravarthi, S. , and Bulleid, N. J. , Glutathione is required to regulate the formulation of native disulfide bonds within proteins entering the secretory pathway, J Biol Chem (2004); 279(38): 39872 -39879. 68. Demitrack, M. A. , Dale, J. K. , Straus, S. E. , Laue, L. , Listwak, S. J. , and Kruesi, M. J. , Evidence for impaired activation of the hypothalamic-pituitary-adrenal axis in patients with chronic fatigue syndrome, J. Clin. Endocrinol. Metab. (1991); 73(6): 124 1234. 69. Bakheit, A. M. , Behan, P. O. , Watson, W. S. , and Morton, J. J. , Abnormal arginine-vasopressin secretion and water metabolism in patients with postviral fatigue syndrome, Acta Neurol. Scand. (1993); 87 (3): 234 -238. 70. Maher, K. J. , Klimas, N. G. and Fletcher, M. A. , Chronic fatigue syndrome is associated with diminished intracellular perforin, Clin. Exp. Immunol. (2005); 142 (3): 505 -511.

References (continued) 71. Kerr, J. , Burke, B. , Petty, R. , Gough, J. , Fear, D. , David, M. , Axford, J. , Dalgleish, A. , and Nutt, D. , Seven genomic subtypes of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME): a detailed analysis of gene networks and clinical phenotypes, J. Clin. Pathol. published online 5 Dec 2007; doi: 10. 1136/jcp. 2007. 053553. 72. Tomoda, A. , Miike, T. , Yamada, E. , Honda, H. , Moroi, T. , Ogawa, M. , Ohtani, Y. , and Morishita, S. , Chronic fatigue syndrome in childhood, Brain & Development (2000); 22: 60 -64. 73. Puri, B. K. , Counsell, S. J. , Saman, R. , Main, J. , Collins, A. G. , Hajnal, J. V. and Davey, N. J. , Relative increase in choline in the occipital cortex in chronic fatigue syndrome, Acta Psychiatr. Scand. (2002); 106: 224 -226. 74. Chaudhuri, A. , Condon, B. R. , Gow, J. W. , Brennan, D. and Hadley, D. M. , Proton magnetic resonance spectroscopy of basal ganglia in chronic fatigue syndrome, Neuro. Report 2003; 14 (2): 225 -228. 75. Levine, S. , Cheney, P. , Shungu, D. C. , and Mao, X. , Analysis of the metabolic features of chronic fatigue syndrome (CFS) using multislice 1 H MRSI, abstract, conference syllabus, Seventh Intl. AACFS Conference on Chronic Fatigue Syndrome, Fibromyalgia and Other Related Illnesses, Madison, WI, U. S. A. , October 8 10, 2004. 76. Kuratsune, H, Yamaguti, K, Takahashi, M. , Misaki, H. , Tagawa, S. , and Kitani, T. , Acylcarnitine deficiency in chronic fatigue syndrome, Clinical Infectious Diseases (1994); 18(Suppl. ): S 62 -S 67. 77. Langsjoen, P. H. , Langsjoen, P. H. and Folkers, K. , Clin. Investig. (1993); 71(8 Suppl): S 140 -S 144. 78. Michiels, V. , and Cluydts, R. , Neuropsychological functioning in chronic fatigue syndrome: a review, Acta Psychiatr Scand. 2001 Feb; 103(2): 84 -93.

Folate metabolism
 Fibromyalgia vs chronic fatigue
Fibromyalgia vs chronic fatigue Methylation vs acetylation
Methylation vs acetylation Methylation & chip-on-chip microarray platform
Methylation & chip-on-chip microarray platform Herzig meyer method reagent
Herzig meyer method reagent Adenine methylation
Adenine methylation Priesthood keys the restoration of priesthood keys
Priesthood keys the restoration of priesthood keys Keys to literacy keys to content writing
Keys to literacy keys to content writing Ischemic heart disease classification
Ischemic heart disease classification Rapoport luebering pathway
Rapoport luebering pathway Parts of a venipuncture needle
Parts of a venipuncture needle Glutathione iv dubai
Glutathione iv dubai Lifewave
Lifewave Tationil glutathione injection in dubai
Tationil glutathione injection in dubai Energy expenditure and fatigue
Energy expenditure and fatigue Age fatigue inattentiveness eyesight and footwear are
Age fatigue inattentiveness eyesight and footwear are Many fears are born of fatigue and loneliness
Many fears are born of fatigue and loneliness Crashworthiness course
Crashworthiness course Compassion fatigue signs
Compassion fatigue signs Fatigue management toolbox talk
Fatigue management toolbox talk Cost of caregiving
Cost of caregiving Fatigue assessment tool
Fatigue assessment tool Fatigue beach marks
Fatigue beach marks What is predominant energy system
What is predominant energy system Ted talk compassion fatigue
Ted talk compassion fatigue Gerber equation fatigue
Gerber equation fatigue Steinberg fatigue
Steinberg fatigue Employee fatigue training
Employee fatigue training Monitor alarm fatigue an integrative review
Monitor alarm fatigue an integrative review Tu es fatigué
Tu es fatigué 30m sprint fatigue test
30m sprint fatigue test Fatigue analysis
Fatigue analysis System shock fatigue
System shock fatigue Fatigue toolbox talk
Fatigue toolbox talk National driver work diary daily sheet
National driver work diary daily sheet Composite fatigue
Composite fatigue Fatigue test
Fatigue test Muscle fatigue
Muscle fatigue Slides fatigue test
Slides fatigue test Alteration de l'etat general selon l'oms
Alteration de l'etat general selon l'oms Fatigue
Fatigue Fatigue
Fatigue Fatigue risk management system template
Fatigue risk management system template Fatigue risk assessment matrix
Fatigue risk assessment matrix On-site manager fatigue workshop
On-site manager fatigue workshop Abs fatigue
Abs fatigue Vulnerability fatigue
Vulnerability fatigue Fatigue template
Fatigue template Appendix
Appendix Thermo-mechanical fatigue
Thermo-mechanical fatigue Objective of stress management
Objective of stress management Defination of fatigue
Defination of fatigue What is compassion fatigue
What is compassion fatigue Corrections fatigue
Corrections fatigue Fatigue crack
Fatigue crack Qqqnn
Qqqnn Durability testing definition
Durability testing definition Fatigue
Fatigue