Methane Pentane Ethane Propane Butane Hexane Notes All

Methane Pentane Ethane Propane Butane Hexane Notes, All the following compounds are Alkanes and end in ane. What is the rule for naming organic compounds?
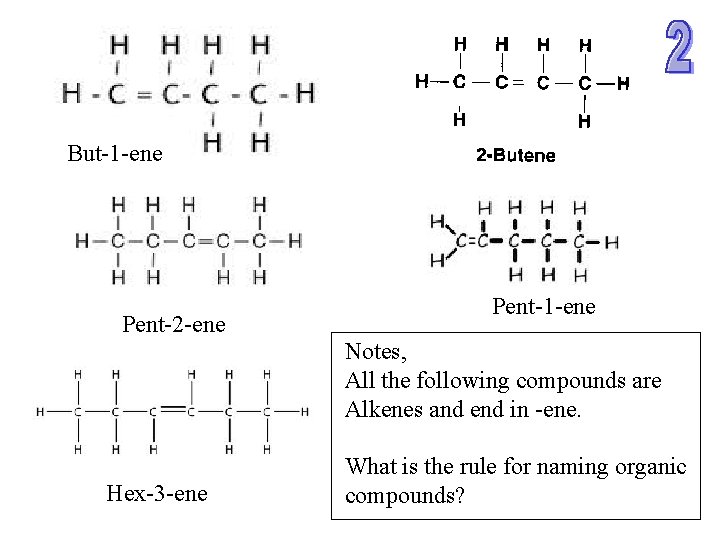
But-1 -ene Pent-2 -ene Pent-1 -ene Notes, All the following compounds are Alkenes and end in -ene. Hex-3 -ene What is the rule for naming organic compounds?
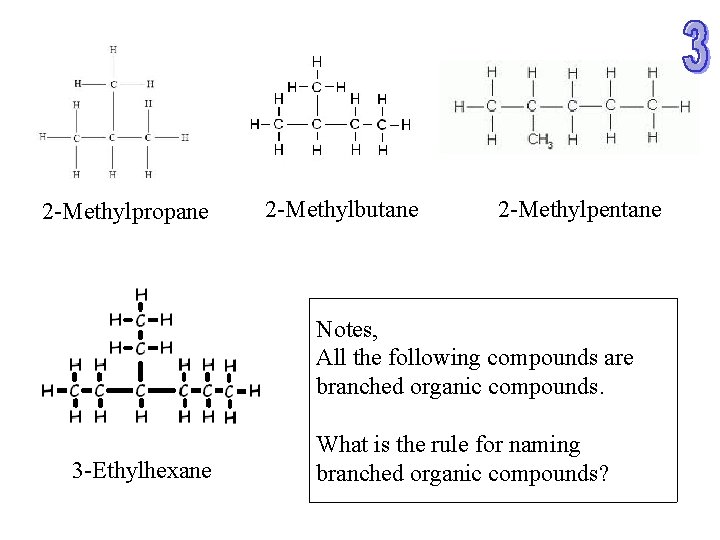
2 -Methylpropane 2 -Methylbutane 2 -Methylpentane Notes, All the following compounds are branched organic compounds. 3 -Ethylhexane What is the rule for naming branched organic compounds?
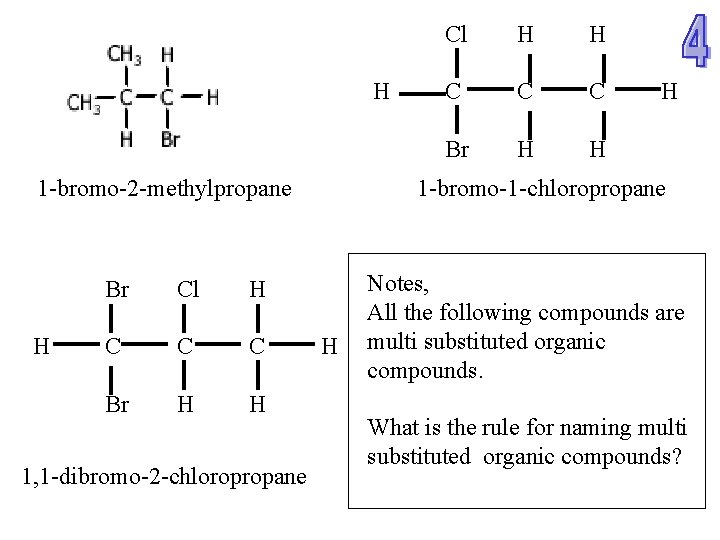
H 1 -bromo-2 -methylpropane H Br Cl H C C C Br H H 1, 1 -dibromo-2 -chloropropane Cl H H C C C Br H H H 1 -bromo-1 -chloropropane H Notes, All the following compounds are multi substituted organic compounds. What is the rule for naming multi substituted organic compounds?
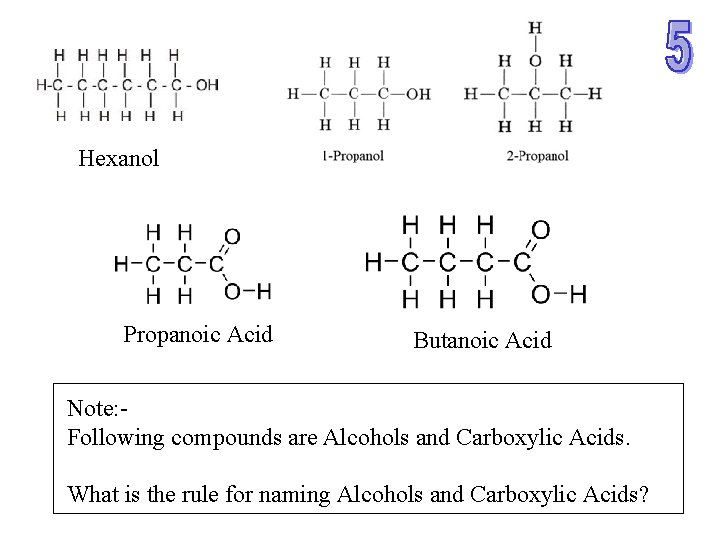
Hexanol Propanoic Acid Butanoic Acid Note: Following compounds are Alcohols and Carboxylic Acids. What is the rule for naming Alcohols and Carboxylic Acids?
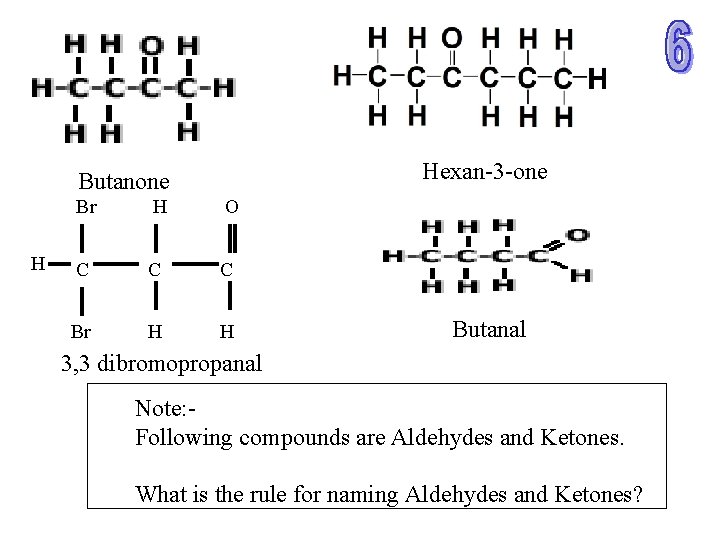
Hexan-3 -one Butanone H Br H O C C C Br H H Butanal 3, 3 dibromopropanal Note: Following compounds are Aldehydes and Ketones. What is the rule for naming Aldehydes and Ketones?
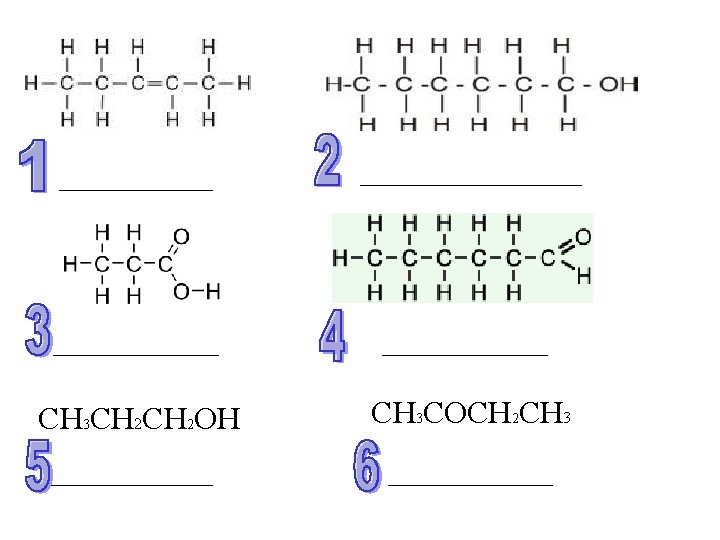
CH 3 CH 2 OH CH 3 COCH 2 CH 3

Answers D/C 1) Pent-2 -ene 2) Hexan-1 -ol 3) Propanoic Acid 4) Hexanal 5) Propan-1 -ol 6) Butanone
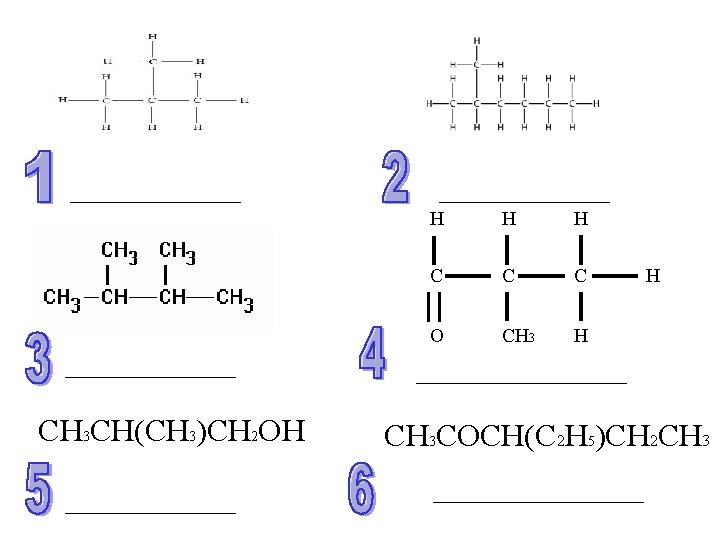
CH 3 CH(CH 3)CH 2 OH H C C C O CH 3 H H CH 3 COCH(C 2 H 5)CH 2 CH 3

Answers C/B 1) 2) 3) 4) 5) 6) 2 -methylpropane 2 -methylhexane 1, 2 -dimethylbutane 2 -methylpropanal 2 -methylpropan-1 -ol 3 -ethylpropentan-2 -one
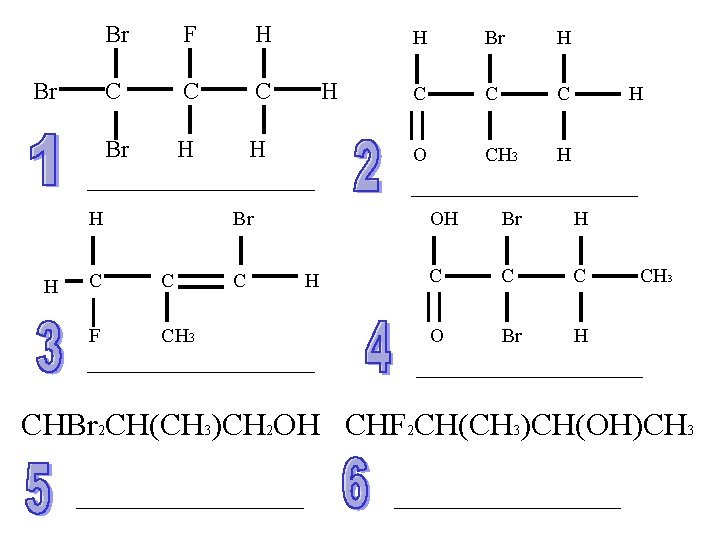
Br Br F H C C C Br H H H Br C C F CH 3 C H H Br H C C C O CH 3 H H OH Br H C C C O Br H CH 3 CHBr 2 CH(CH 3)CH 2 OH CHF 2 CH(CH 3)CH(OH)CH 3

Answers B/A 1) 1, 1, 1 tribromo-2 -fluropropane 2) 2 -bromo-2 -methylpropanal 3) 1 -bromo-3 -fluro-2 -methylprop-1 -ene 4) 2, 2 -dibromobutanoic acid 5) 3, 3 -dibromo-2 -methylpropan-1 -ol 6) 4, 4 -difluro-3 -methylbutan-2 -ol
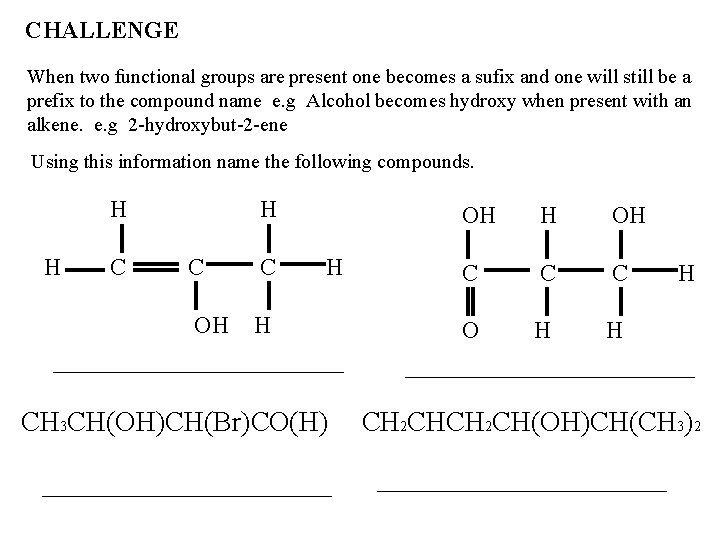
CHALLENGE When two functional groups are present one becomes a sufix and one will still be a prefix to the compound name e. g Alcohol becomes hydroxy when present with an alkene. e. g 2 -hydroxybut-2 -ene Using this information name the following compounds. H H C C OH H H CH 3 CH(OH)CH(Br)CO(H) OH H OH C C C O H H H CH 2 CH(OH)CH(CH 3)2

CHALLENGE 1) 2 -hydroxyprop-1 -ene 2) 2 -hydroxypropanal 3) 2 -bromo-3 -hydroxybutanal 4) 4 -hydroxy-5 -methylhex-1 -ene
- Slides: 14