Methane Hydrates Zack Fink The Methane Hydrate What











- Slides: 14

Methane Hydrates Zack Fink

The Methane Hydrate: What is it?

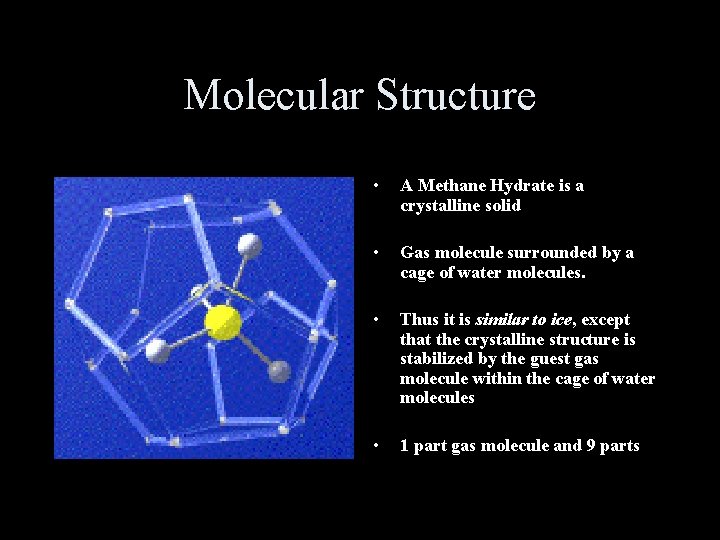
Molecular Structure • A Methane Hydrate is a crystalline solid • Gas molecule surrounded by a cage of water molecules. • Thus it is similar to ice, except that the crystalline structure is stabilized by the guest gas molecule within the cage of water molecules • 1 part gas molecule and 9 parts water

Formation and Function • Concentrates methane gas, 150 -180 x more • Form in pore spaces of sediment • Causes cementation of sediment • Cemented sediment often acts as a cap on pure gas deposits Form in high pressure / low temperature environments, in ocean this translates to seafloor depths of 500+ meters. Also found on land in permafrost. Other limiting factors are methane and sediment. Most methane comes from bacterial breakdown of organic detritus in aneorbic conditions. So, hydrate typically forms in areas with abundance of organic material and sediment to cover and protect from oxidation

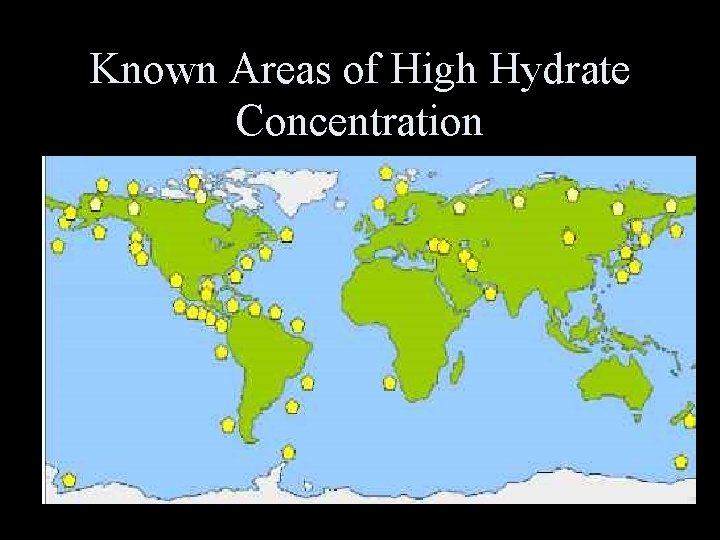
Known Areas of High Hydrate Concentration


Methane Hydrates: …So What?



Hydrate Issues • Good • Immense potential for use as a relatively environmentally friendly energy source • Bad • Greenhouse effect /global warming /sea level rise • Submarine Slumping • Oil pipeline interference

Global Climate Change • Methane is a particularly nasty green house gas • 10 x more effective than CO 2 • Continually seeps into atmosphere • There is clearly a lot, the potential for serious environmental impacts is great • Real Problem is the chain reaction • Hydrate is a frozen solid, so the hotter the world gets, the more methane disassociates out of the hydrate, making the world even hotter

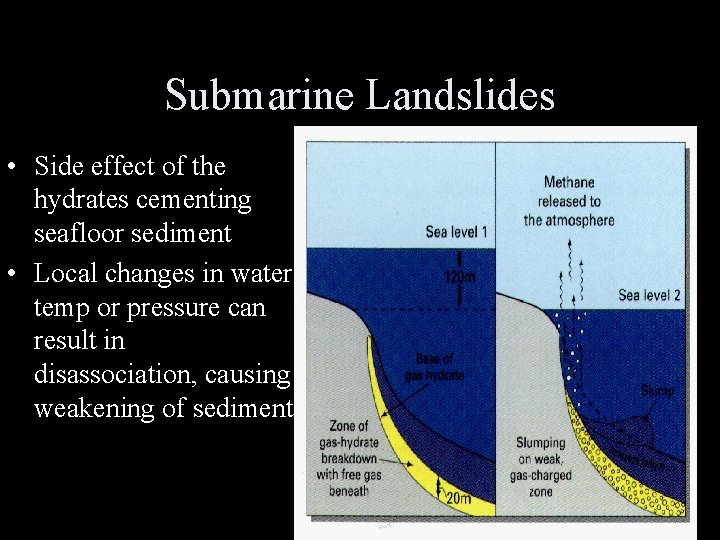
Submarine Landslides • Side effect of the hydrates cementing seafloor sediment • Local changes in water temp or pressure can result in disassociation, causing weakening of sediment.

Efforts at Exploitation Currently, economic extraction is not feasible Research on large scale extraction is in its infancy No obvious best method of extraction has been agreed upon • Proposed Plans • Mining existing hydrate and transporting in pipes • Growing Hydrates from methane seeps • Transportation by submarine towing bladders • Chemical additives making hydrates stable at lower pressures and higher temperatures

Yes or No? • Methane Hydrates could turn out to be a tremendous natural resource • Or they could prove too cumbersome to exploit… • Either way, research is not an option • Methane hydrates are far from completely understood, but one thing is certain, they are incredibly important • Through research there exists a possibility to turn a potentially leading cause of global warming into a valued extension of the fossil fuel economy