METATHESIS REACTIONS DOUBLE REPLACEMENT The driving force All

METATHESIS REACTIONS DOUBLE REPLACEMENT

The driving force: All double replacement reactions must have a “driving force” or reason why the reaction will go to completion. This is the removal of at least one pair of ions from solution Driving forces: Gas Formation (learn common ones) Electrolytes (formation of weak/non-electrolytes) Precipitation (learn solubility rules)

H 2 S: Any sulfide (salt of S-2) plus Common Gases Look for trends, memorize! and acid form H 2 S(g) and a salt. CO 2: Any carbonate (salt of CO 32 -) plus any acid form CO 2(g) , water and salt. SO 2: Any sulfite (salt of SO 3 -2)plus any acid form SO 2(g), water and salt. NH 3: Any ammonium salt plus any soluble strong hydroxide react upon heating to form NH 3(g), water and salt.
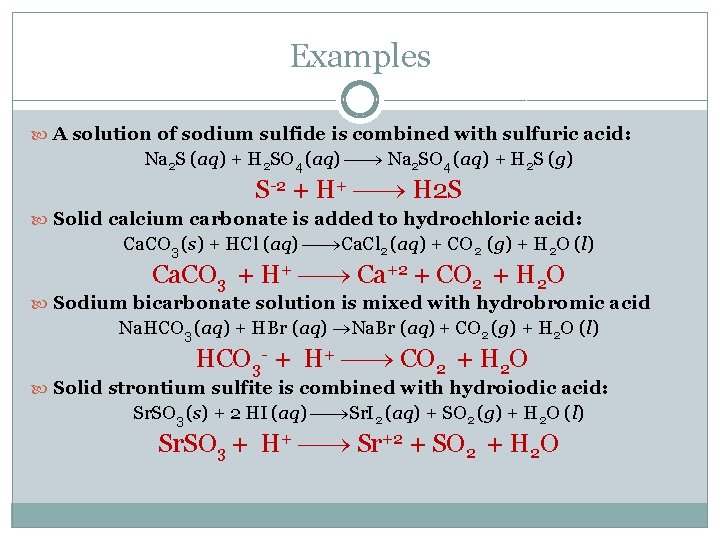
Examples A solution of sodium sulfide is combined with sulfuric acid: Na 2 S (aq) + H 2 SO 4 (aq) Na 2 SO 4 (aq) + H 2 S (g) S-2 + H+ H 2 S Solid calcium carbonate is added to hydrochloric acid: Ca. CO 3 (s) + HCl (aq) Ca. Cl 2 (aq) + CO 2 (g) + H 2 O (l) Ca. CO 3 + H+ Ca+2 + CO 2 + H 2 O Sodium bicarbonate solution is mixed with hydrobromic acid Na. HCO 3 (aq) + HBr (aq) Na. Br (aq) + CO 2 (g) + H 2 O (l) HCO 3 - + H+ CO 2 + H 2 O Solid strontium sulfite is combined with hydroiodic acid: Sr. SO 3 (s) + 2 HI (aq) Sr. I 2 (aq) + SO 2 (g) + H 2 O (l) Sr. SO 3 + H+ Sr+2 + SO 2 + H 2 O

Electrolytes A strong electrolyte dissociates completely when dissolved in water. A weak electrolyte only dissociates partially when dissolved in water.

Acids There are only seven strong acids: • • Hydrochloric (HCl) Hydrobromic (HBr) Hydroiodic (HI) Nitric (HNO 3) Sulfuric (H 2 SO 4) Chloric (HCl. O 3) Perchloric (HCl. O 4)

Bases: Substances that increase the concentration of OH− when dissolved in water (Arrhenius). Proton acceptors (Brønsted–Lowry).
Bases The strong bases are the soluble salts of hydroxide ion: • • Alkali metals Calcium Strontium Barium
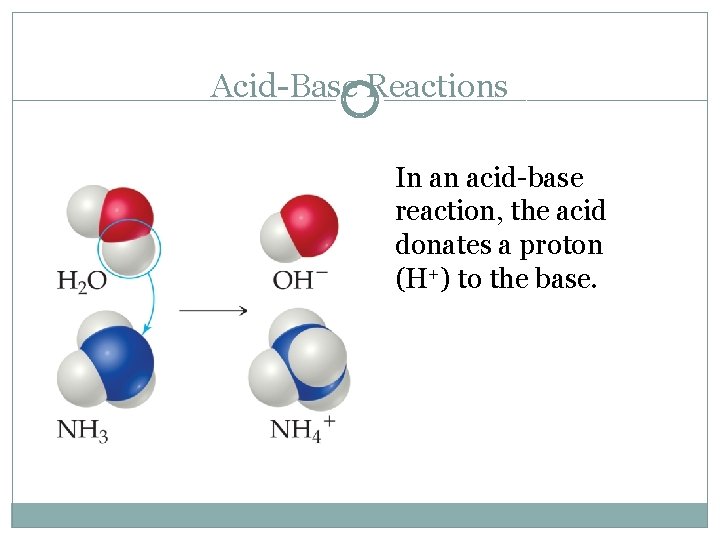
Acid-Base Reactions In an acid-base reaction, the acid donates a proton (H+) to the base.
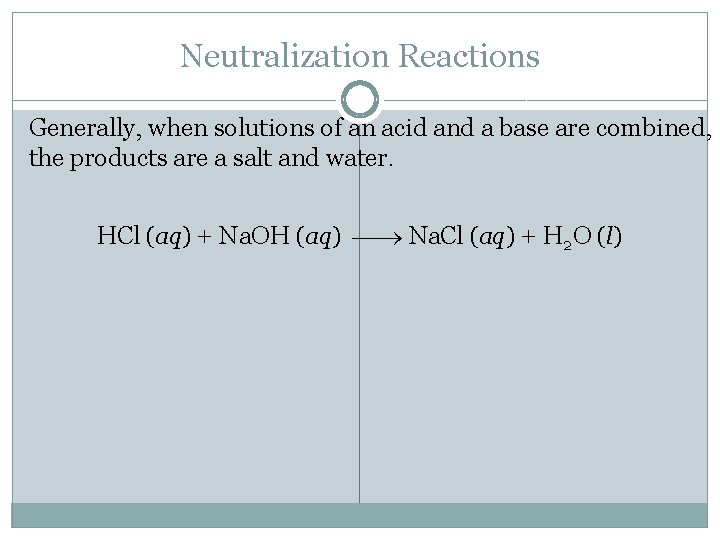
Neutralization Reactions Generally, when solutions of an acid and a base are combined, the products are a salt and water. HCl (aq) + Na. OH (aq) Na. Cl (aq) + H 2 O (l)
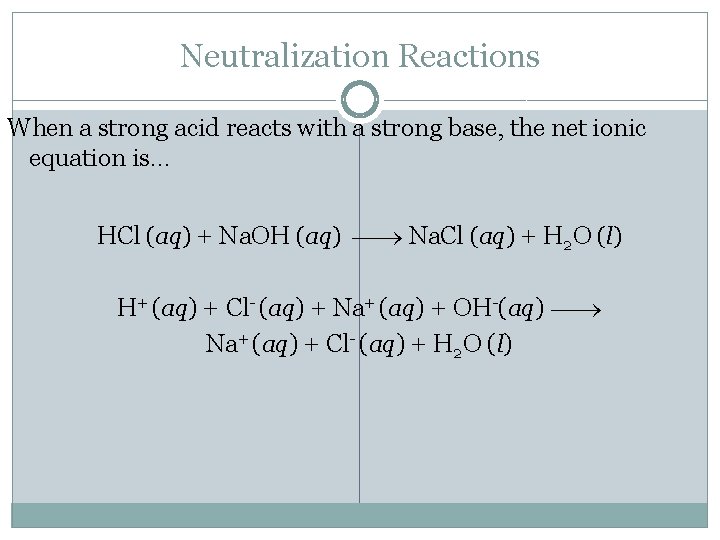
Neutralization Reactions When a strong acid reacts with a strong base, the net ionic equation is… HCl (aq) + Na. OH (aq) Na. Cl (aq) + H 2 O (l) H+ (aq) + Cl- (aq) + Na+ (aq) + OH-(aq) Na+ (aq) + Cl- (aq) + H 2 O (l)
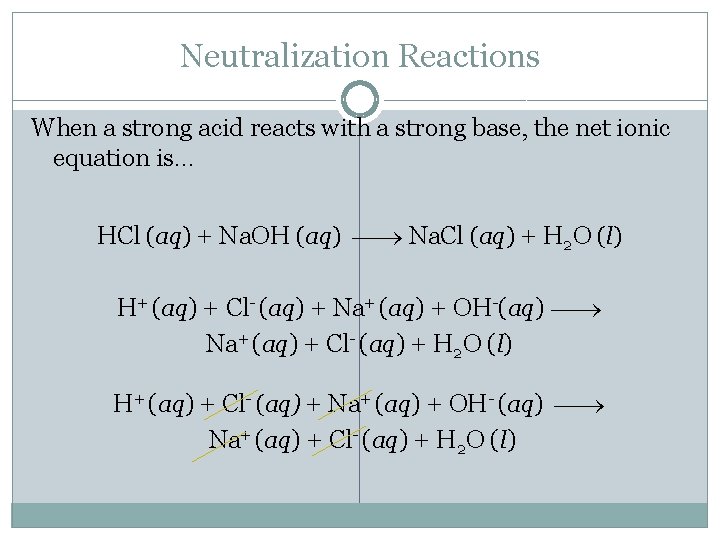
Neutralization Reactions When a strong acid reacts with a strong base, the net ionic equation is… HCl (aq) + Na. OH (aq) Na. Cl (aq) + H 2 O (l) H+ (aq) + Cl- (aq) + Na+ (aq) + OH-(aq) Na+ (aq) + Cl- (aq) + H 2 O (l) H+ (aq) + Cl- (aq) + Na+ (aq) + OH- (aq) Na+ (aq) + Cl- (aq) + H 2 O (l)
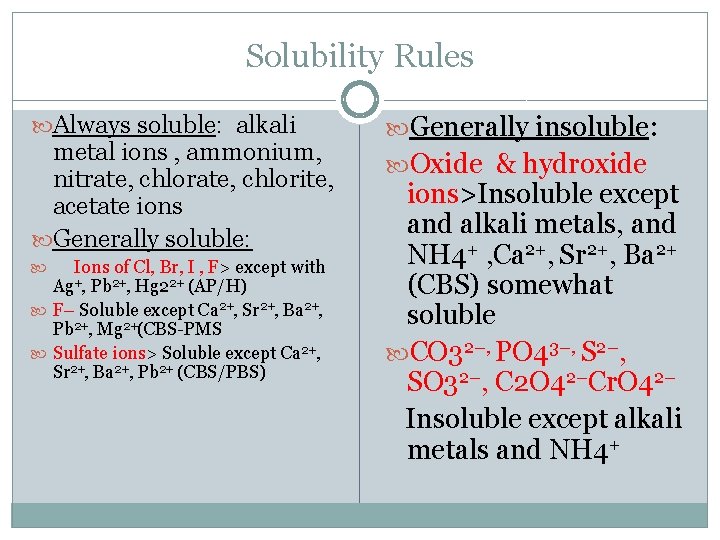
Solubility Rules Always soluble: alkali metal ions , ammonium, nitrate, chlorite, acetate ions Generally soluble: Ions of Cl, Br, I , F> except with Pb 2+, Hg 22+ (AP/H) F– Soluble except Ca 2+, Sr 2+, Ba 2+, Pb 2+, Mg 2+(CBS-PMS Sulfate ions> Soluble except Ca 2+, Sr 2+, Ba 2+, Pb 2+ (CBS/PBS) Ag+, Generally insoluble: Oxide & hydroxide ions>Insoluble except and alkali metals, and NH 4+ , Ca 2+, Sr 2+, Ba 2+ (CBS) somewhat soluble CO 32–, PO 43–, S 2–, SO 32–, C 2 O 42–Cr. O 42– Insoluble except alkali metals and NH 4+
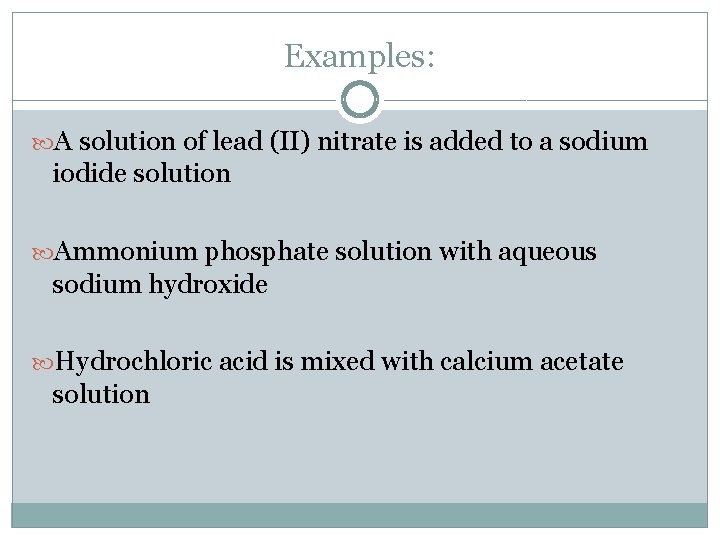
Examples: A solution of lead (II) nitrate is added to a sodium iodide solution Ammonium phosphate solution with aqueous sodium hydroxide Hydrochloric acid is mixed with calcium acetate solution
- Slides: 14