Metastatic Renal Cell Carcinoma Whats Hot In The














- Slides: 37

Metastatic Renal Cell Carcinoma What’s Hot In The Treatment Of Renal Cell Carcinoma And Is There Hope? Cora N. Sternberg, MD, FACP Chairman, Department of Medical Oncology San Camillo and Forlanini Hospitals Rome, Italy

Metastatic Kidney Cancer: Options • Interferon- for good risk patients until recently • HD-IL-2 for intermediate and good risk patients. – Limited availability – Intensive treatment – Of value (long lasting CR) for a small group of patients – Patient selection required • Poor risk patients: no proven therapeutic options until recently • Second line: no proven therapeutic options until recently

Treatment of Metastatic Kidney Cancer • Has our perception of RCC been changed with the advent of new drugs? • Can we commute a death sentence to a chronic disease that patients can learn to live with? • How have traditional criteria to measure response with cytotoxics led us astray? • Can we afford these expensive promising new treatments? Mancuso and Sternberg, BJU Int. Jun 2005 Mancuso and Sternberg, Can J Urol. Feb 2005

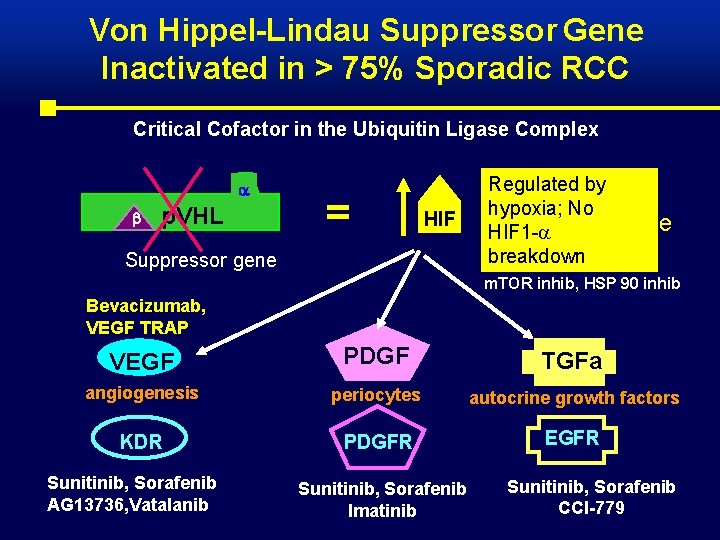
Von Hippel-Lindau Suppressor Gene Inactivated in > 75% Sporadic RCC Critical Cofactor in the Ubiquitin Ligase Complex p. VHL = HIF Suppressor gene Regulated by degradation of hypoxia; No hypoxia HIF 1 -a inducible breakdown m. TOR inhib, HSP 90 inhib Bevacizumab, VEGF TRAP VEGF PDGF TGFa angiogenesis periocytes autocrine growth factors KDR PDGFR EGFR Sunitinib, Sorafenib AG 13736, Vatalanib Sunitinib, Sorafenib Imatinib Sunitinib, Sorafenib CCI-779

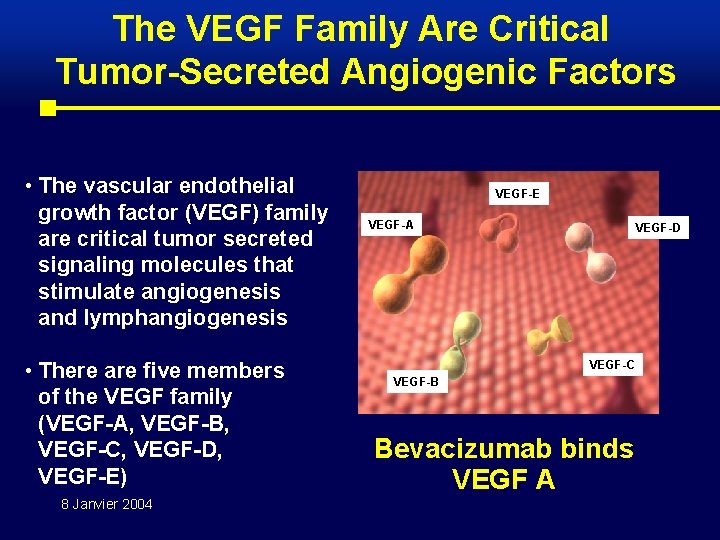
The VEGF Family Are Critical Tumor-Secreted Angiogenic Factors • The vascular endothelial growth factor (VEGF) family are critical tumor secreted signaling molecules that stimulate angiogenesis and lymphangiogenesis • There are five members of the VEGF family (VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E) 8 Janvier 2004 VEGF-E VEGF-A VEGF-D VEGF-C VEGF-B Bevacizumab binds VEGF A

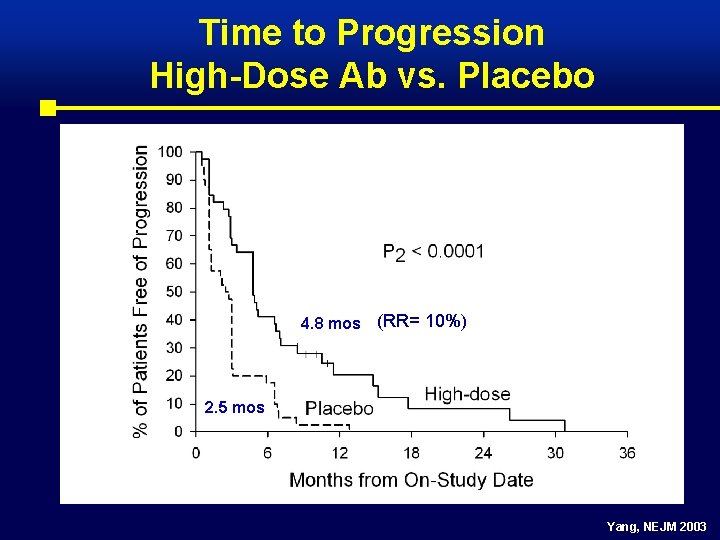
Time to Progression High-Dose Ab vs. Placebo 4. 8 mos (RR= 10%) 2. 5 mos Yang, NEJM 2003

Survival Update Yang, NEJM 2003

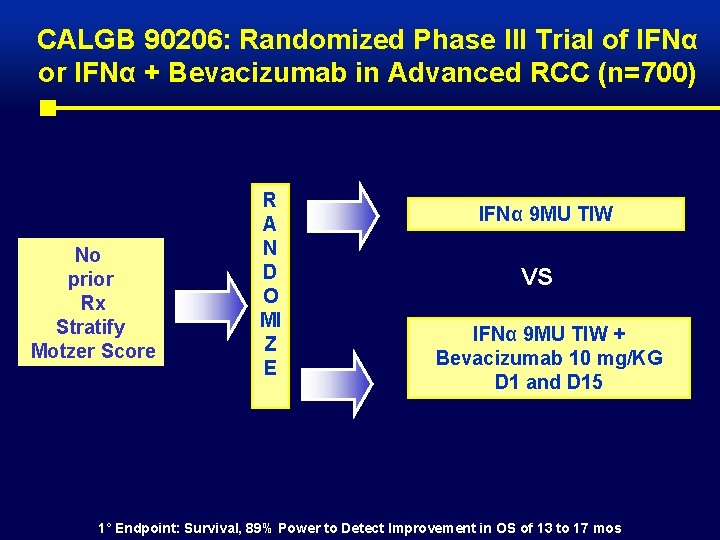
CALGB 90206: Randomized Phase III Trial of IFNα or IFNα + Bevacizumab in Advanced RCC (n=700) No prior Rx Stratify Motzer Score R A N D O MI Z E IFNα 9 MU TIW VS IFNα 9 MU TIW + Bevacizumab 10 mg/KG D 1 and D 15 1° Endpoint: Survival, 89% Power to Detect Improvement in OS of 13 to 17 mos

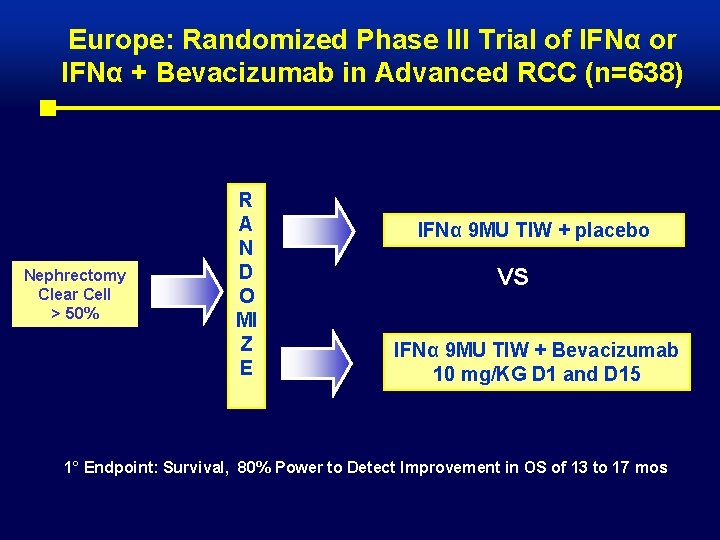
Europe: Randomized Phase III Trial of IFNα or IFNα + Bevacizumab in Advanced RCC (n=638) Nephrectomy Clear Cell > 50% R A N D O MI Z E IFNα 9 MU TIW + placebo VS IFNα 9 MU TIW + Bevacizumab 10 mg/KG D 1 and D 15 1° Endpoint: Survival, 80% Power to Detect Improvement in OS of 13 to 17 mos

Sunitinib Mechanism of Action in RCC Loss of VHL Protein Function ↑ VEGF ↑ PDGF VEGF PDGF Vascular Endothelial Cell Pericyte/Fibroblast/ Vascular Smooth Muscle VEGFR PDGFR Sunitinib Vascular permeability Cell survival, proliferation, migration Vascular formation, maturation Inhibition of RCC pathogenesis and progression

Best Response By RECIST Response Trial 1 n (%) Trial 2 n (%) Patients 63 106 Overall response Complete response Partial response 25 (40) 0 25 (40) 44 (42) 1 (1) 43 (41) Stable disease (SD) 3 months 18 (28) 22 (21) Progression, SD <3 months 16 (25) 33 (31) Not evaluable 4 (6) 7 (7) Motzer R , J Clin Oncol. 2006 Jan 1; 24(1): 16 -24 Motzer R, JAMA. 2006 Jun 7; 295(21): 2516 -24

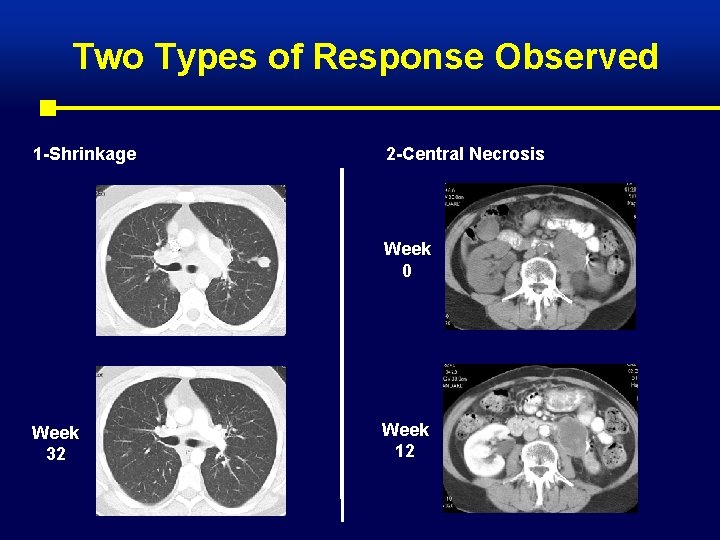
Two Types of Response Observed 1 -Shrinkage 2 -Central Necrosis Week 0 Week 32 Week 12

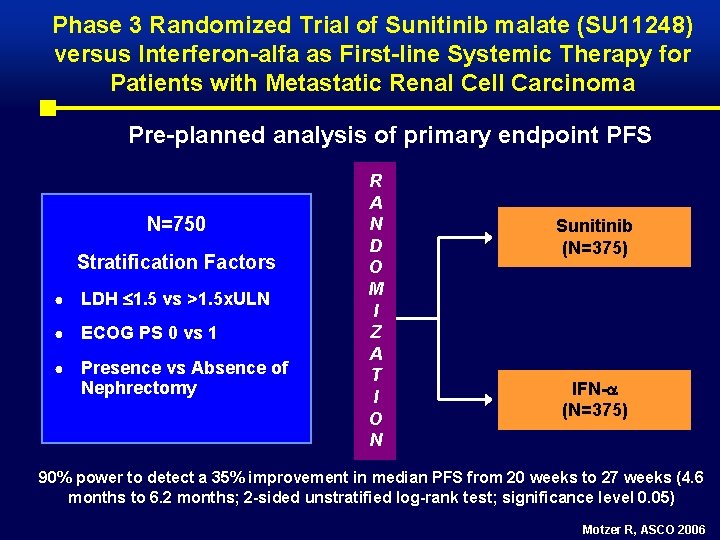
Phase 3 Randomized Trial of Sunitinib malate (SU 11248) versus Interferon-alfa as First-line Systemic Therapy for Patients with Metastatic Renal Cell Carcinoma Pre-planned analysis of primary endpoint PFS N=750 Stratification Factors ● LDH 1. 5 vs >1. 5 x. ULN ● ECOG PS 0 vs 1 ● Presence vs Absence of Nephrectomy R A N D O M I Z A T I O N Sunitinib (N=375) IFN- (N=375) 90% power to detect a 35% improvement in median PFS from 20 weeks to 27 weeks (4. 6 months to 6. 2 months; 2 -sided unstratified log-rank test; significance level 0. 05) Motzer R, ASCO 2006

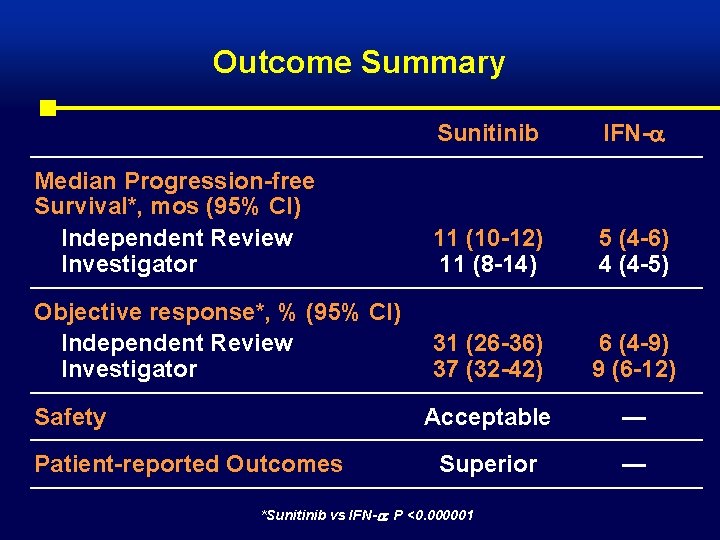
Outcome Summary Sunitinib IFN- Median Progression-free Survival*, mos (95% CI) Independent Review Investigator 11 (10 -12) 11 (8 -14) 5 (4 -6) 4 (4 -5) Objective response*, % (95% CI) Independent Review Investigator 31 (26 -36) 37 (32 -42) 6 (4 -9) 9 (6 -12) Acceptable — Superior — Safety Patient-reported Outcomes *Sunitinib vs IFN- : P <0. 000001

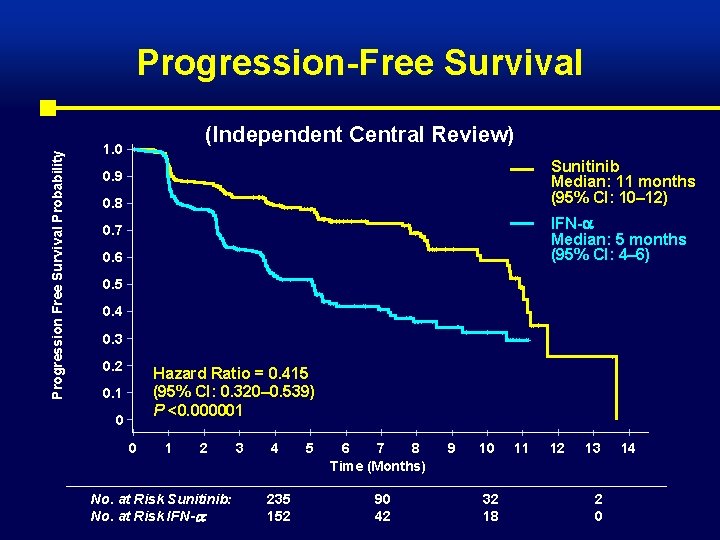
Progression Free Survival Probability Progression-Free Survival (Independent Central Review) 1. 0 Sunitinib Median: 11 months (95% CI: 10– 12) 0. 9 0. 8 IFN- Median: 5 months (95% CI: 4– 6) 0. 7 0. 6 0. 5 0. 4 0. 3 0. 2 Hazard Ratio = 0. 415 (95% CI: 0. 320– 0. 539) P <0. 000001 0. 1 0 0 1 2 No. at Risk Sunitinib: No. at Risk IFN- : 3 4 235 152 5 6 7 8 Time (Months) 90 42 9 10 32 18 11 12 13 2 0 14

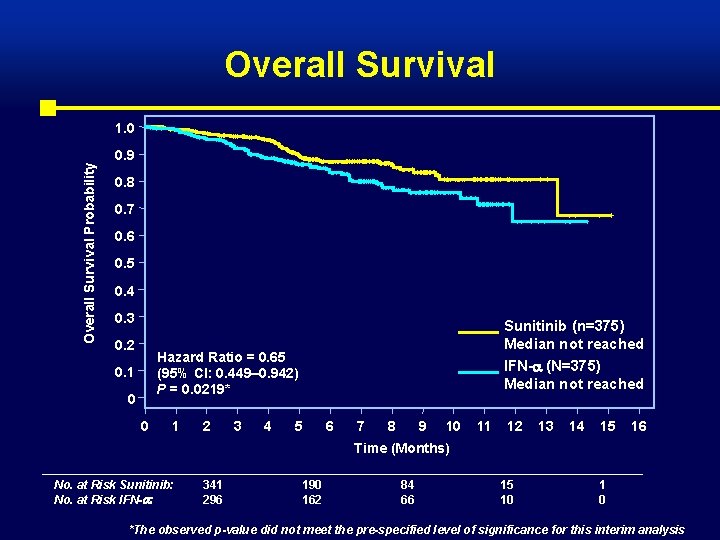
Overall Survival Probability 1. 0 0. 9 0. 8 0. 7 0. 6 0. 5 0. 4 0. 3 0. 2 Sunitinib (n=375) Median not reached Hazard Ratio = 0. 65 (95% CI: 0. 449– 0. 942) P = 0. 0219* 0. 1 0 0 1 2 3 4 IFN- (N=375) Median not reached 5 6 7 8 9 10 11 12 13 14 15 16 Time (Months) No. at Risk Sunitinib: No. at Risk IFN- : 341 296 190 162 84 66 15 10 1 0 *The observed p-value did not meet the pre-specified level of significance for this interim analysis

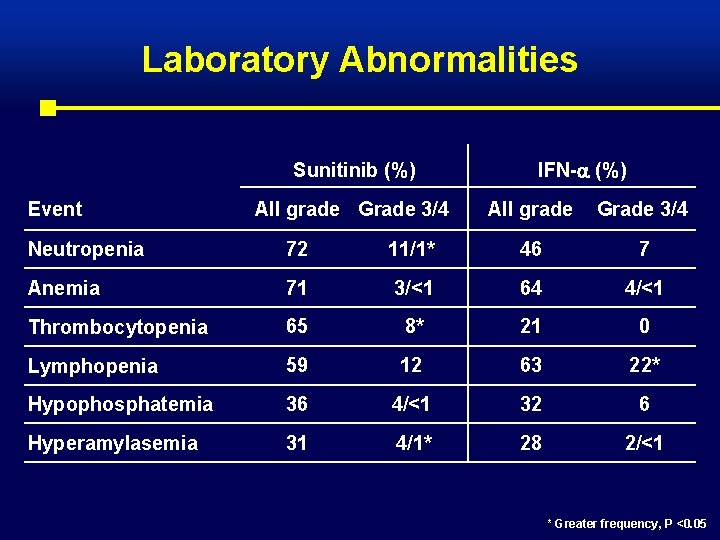
Laboratory Abnormalities Sunitinib (%) Event All grade Grade 3/4 IFN- (%) All grade Grade 3/4 Neutropenia 72 11/1* 46 7 Anemia 71 3/<1 64 4/<1 Thrombocytopenia 65 8* 21 0 Lymphopenia 59 12 63 22* Hypophosphatemia 36 4/<1 32 6 Hyperamylasemia 31 4/1* 28 2/<1 * Greater frequency, P <0. 05

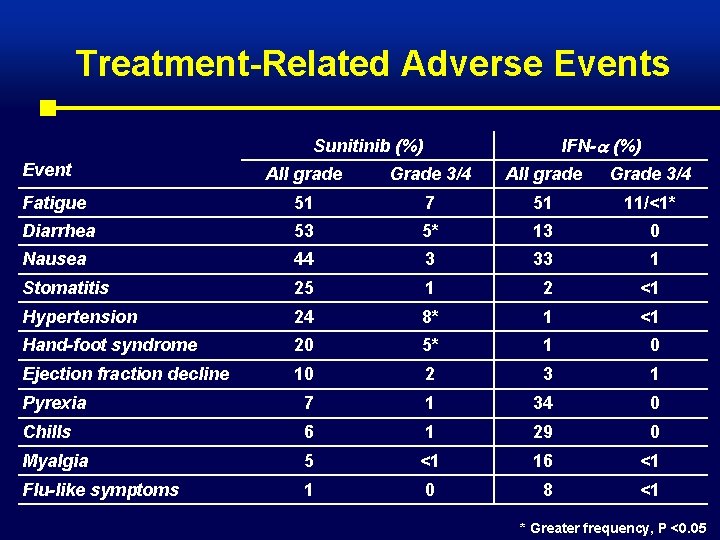
Treatment-Related Adverse Events IFN- (%) Sunitinib (%) Event All grade Grade 3/4 Fatigue 51 7 51 11/<1* Diarrhea 53 5* 13 0 Nausea 44 3 33 1 Stomatitis 25 1 2 <1 Hypertension 24 8* 1 <1 Hand-foot syndrome 20 5* 1 0 Ejection fraction decline 10 2 3 1 Pyrexia 7 1 34 0 Chills 6 1 29 0 Myalgia 5 <1 16 <1 Flu-like symptoms 1 0 8 <1 * Greater frequency, P <0. 05

Conclusions • Sunitinib is a new reference standard for the first-line treatment of RCC • Mechanism-directed RCC therapy based on tumor-specific molecular features is validated • Sunitinib is a new treatment option providing hope for patients with RCC Motzer R, ASCO 2006

Global ARCC Trial A Phase 3, Randomized, 3 -Arm Study of Temsirolimus (TEMSR) or Interferon-Alpha (IFN) or the Combination of TEMSR + IFN in the Treatment of First-Line, Poor-Risk Patients With Advanced Renal Cell Carcinoma G Hudes, M Carducci, P Tomczak, J Dutcher, R Figlin, A Kapoor, E Staroslawska, T O’Toole, S Kong, and L Moore 2006 ASCO Presentation

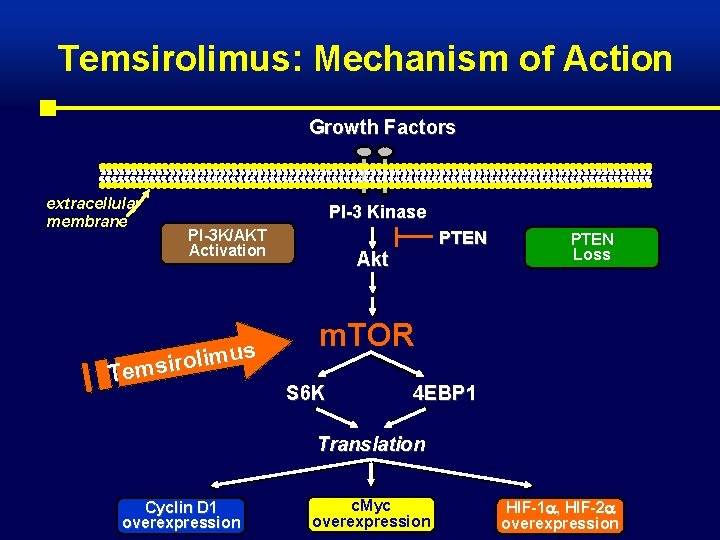
Temsirolimus: Mechanism of Action Growth Factors extracellular membrane PI-3 Kinase PI-3 K/AKT Activation us m i l o r i Tems PTEN Akt PTEN Loss m. TOR S 6 K 4 EBP 1 Translation Cyclin D 1 overexpression c. Myc overexpression HIF-1 , HIF-2 overexpression

Global ARCC Trial Phase 3 Study of TEMSR and IFN in Advanced RCC • 626 patients with advanced metastatic RCC with poorrisk features • 209 sites (26 countries) Stratification by: Geographic Regions: • WEU + AU + CA (22%) • US (30%) • EEU + Other (48%) Nephrectomy: • Yes (67%) • No (33%) R A N D O M I Z E IFN: escalating to 18 MU SC TIW n = 207 TEMSR: 25 mg IV QW n = 209 TEMSR: 15 mg IV QW + IFN: 6 MU TIW n = 210

Overall Survival by Treatment Arm Parameter n IFN Arm 1 TEMSR Arm 2 TEMSR + IFN Arm 3 207 209 210 Arm 2: Arm 1 Arm 3: Arm 1 0. 0069 0. 6912 Comparisons Probability of Survival Stratified Log-Rank p Arm 2: Temsirolimus Arm 1: IFN Arm 3: IFN + Temsirolimus Time from Randomization, Months

Global ARCC Trial Overall Survival by Treatment Arm IFN Arm 1 n=207 TEMSR Arm 2 n=209 TEMSR + IFN Arm 3 n=210 Deaths, n 149 141 152 Median Survival, months (95% CI) 7. 3 (6. 1 - 8. 9) 10. 9 (8. 6 - 12. 7) 8. 4 (6. 6 - 10. 2) Arm 2: Arm 1 Arm 3: Arm 1 Increase in Median Survival 49% 15% Hazard Ratio (95% CI) 0. 73 (0. 57 - 0. 92) 0. 95 (0. 76 - 1. 2) Stratified Log-Rank p 0. 0069* 0. 6912 *O’Brien-Fleming boundary for significance = 0. 0155

Global ARCC Trial • This is the first study to demonstrate a statistically significant improvement in survival in advanced poor-risk RCC patients • The results of this global phase 3 trial demonstrate that m. TOR is an important therapeutic target in RCC

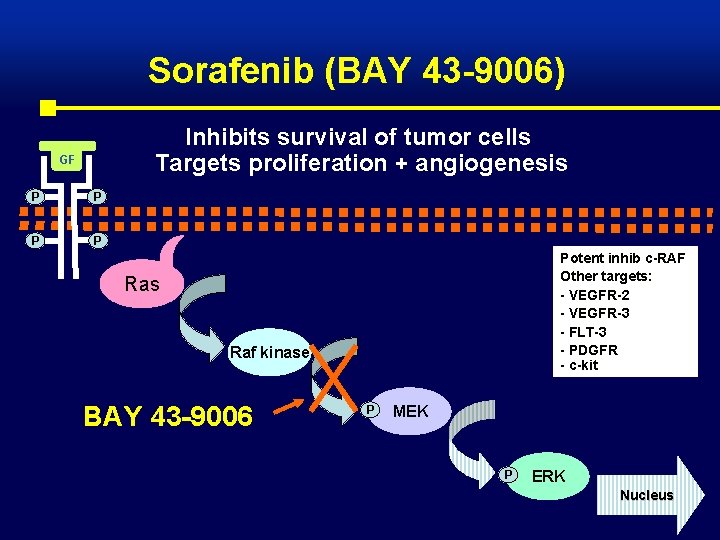
Sorafenib (BAY 43 -9006) Inhibits survival of tumor cells Targets proliferation + angiogenesis GF P P Potent inhib c-RAF Other targets: - VEGFR-2 - VEGFR-3 - FLT-3 - PDGFR - c-kit Ras Raf kinase BAY 43 -9006 P MEK P ERK Nucleus

Sorafenib (BAY 43 -9006) Randomized Discontinuation Trial Schema (n=202) > 25% Shrinkage Continue BAY 43 -9006 Open Label BAY 43 -9006 >-25% to <25% Randomized 12 Week Induction Tumor Assessment Placebo* > 25% Growth Off study Baseline 12 weeks * May cross over to BAY 43 -9006 24 weeks Ratain, ASCO 2005 Eisen T, Br J Cancer. 2006 Sep 4; 95(5): 581 -6

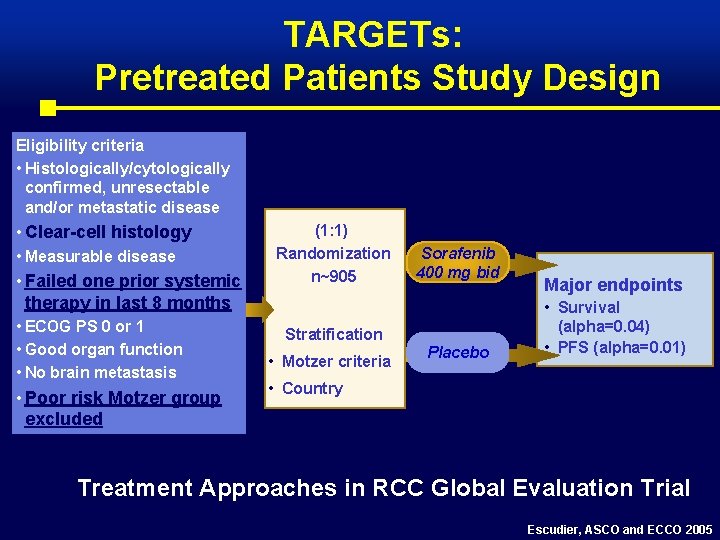
TARGETs: Pretreated Patients Study Design Eligibility criteria • Histologically/cytologically confirmed, unresectable and/or metastatic disease • Clear-cell histology • Measurable disease • Failed one prior systemic therapy in last 8 months • ECOG PS 0 or 1 • Good organ function • No brain metastasis • Poor risk Motzer group excluded (1: 1) Randomization n~905 Sorafenib 400 mg bid Stratification • Motzer criteria Placebo Major endpoints • Survival (alpha=0. 04) • PFS (alpha=0. 01) • Country Treatment Approaches in RCC Global Evaluation Trial Escudier, ASCO and ECCO 2005

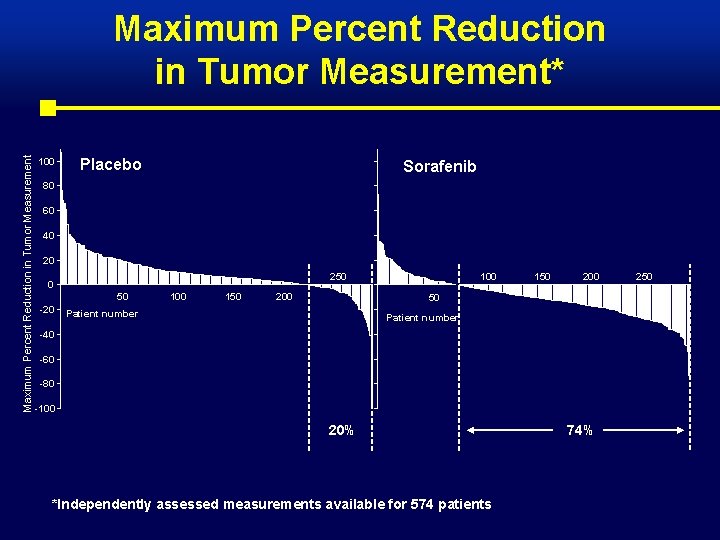
Maximum Percent Reduction in Tumor Measurement* 100 Placebo Sorafenib 80 60 40 20 250 0 50 -20 100 150 200 50 Patient number -40 -60 -80 -100 20% *Independently assessed measurements available for 574 patients 74% 250

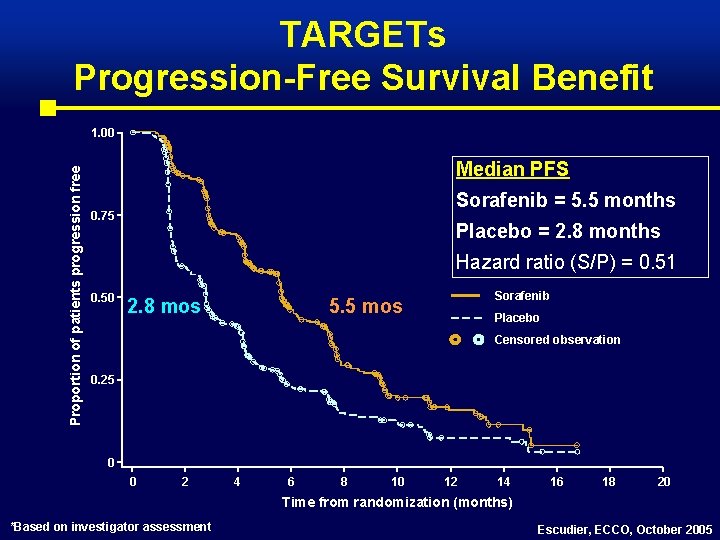
TARGETs Progression-Free Survival Benefit Proportion of patients progression free 1. 00 Median PFS Sorafenib = 5. 5 months 0. 75 Placebo = 2. 8 months Hazard ratio (S/P) = 0. 51 0. 50 2. 8 mos Sorafenib 5. 5 mos Placebo Censored observation 0. 25 0 0 2 4 6 8 10 12 14 16 18 20 Time from randomization (months) *Based on investigator assessment Escudier, ECCO, October 2005

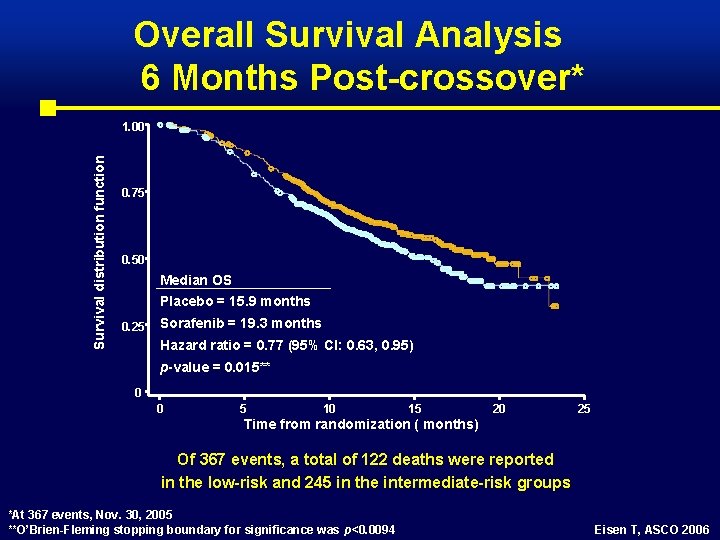
Overall Survival Analysis 6 Months Post-crossover* Survival distribution function 1. 00 0. 75 0. 50 Median OS Placebo = 15. 9 months 0. 25 Sorafenib = 19. 3 months Hazard ratio = 0. 77 (95% CI: 0. 63, 0. 95) p-value = 0. 015** 0 0 5 10 15 20 25 Time from randomization ( months) Of 367 events, a total of 122 deaths were reported in the low-risk and 245 in the intermediate-risk groups *At 367 events, Nov. 30, 2005 **O’Brien-Fleming stopping boundary for significance was p<0. 0094 Eisen T, ASCO 2006

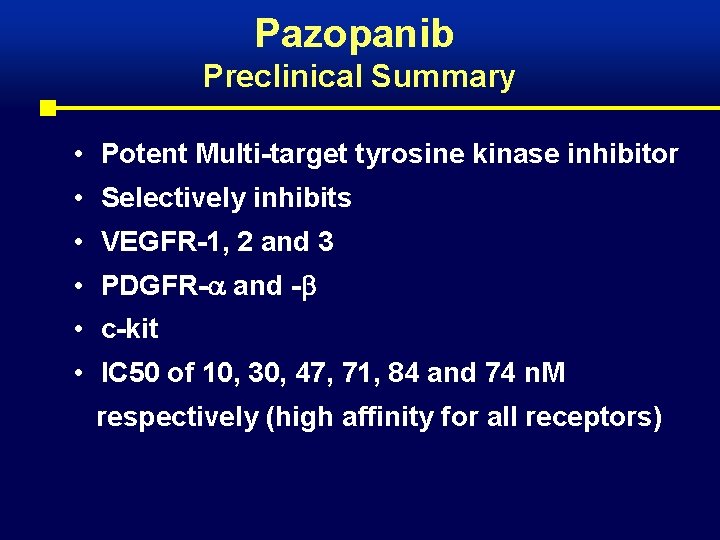
Pazopanib Preclinical Summary • Potent Multi-target tyrosine kinase inhibitor • Selectively inhibits • VEGFR-1, 2 and 3 • PDGFR- and - • c-kit • IC 50 of 10, 30, 47, 71, 84 and 74 n. M respectively (high affinity for all receptors)

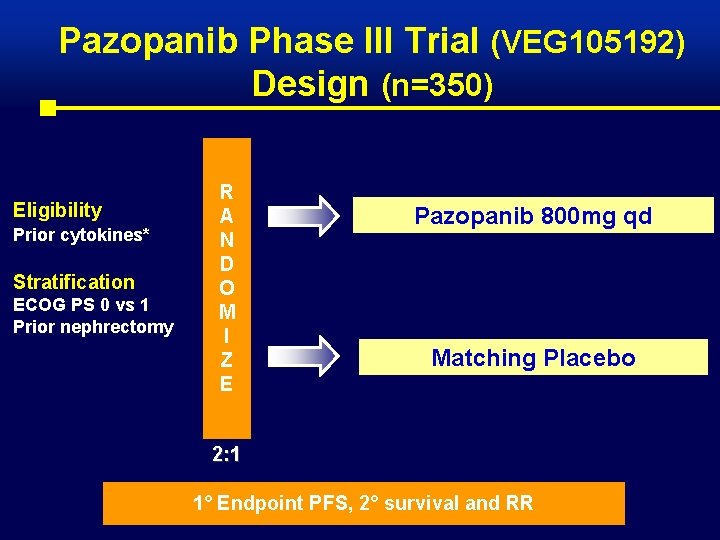
Pazopanib Phase III Trial (VEG 105192) Design (n=350) Eligibility Prior cytokines* Stratification ECOG PS 0 vs 1 Prior nephrectomy R A N D O M I Z E Pazopanib 800 mg qd Matching Placebo 2: 1 1° Endpoint PFS, 2° survival and RR

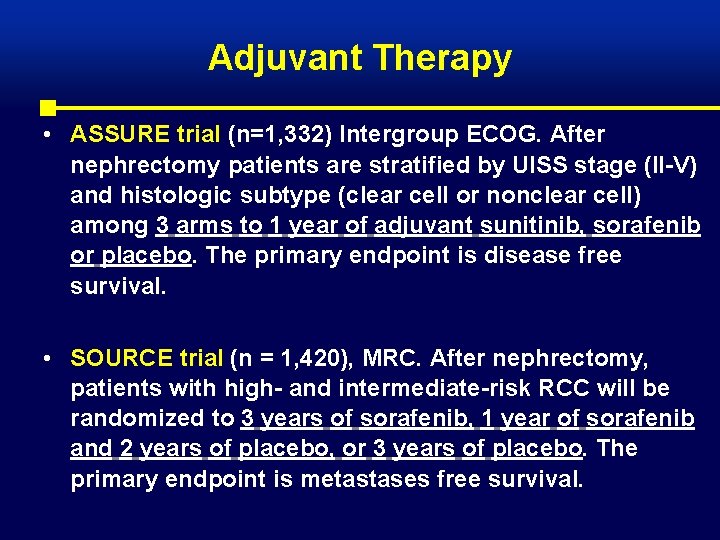
Adjuvant Therapy • ASSURE trial (n=1, 332) Intergroup ECOG. After nephrectomy patients are stratified by UISS stage (II-V) and histologic subtype (clear cell or nonclear cell) among 3 arms to 1 year of adjuvant sunitinib, sorafenib or placebo. The primary endpoint is disease free survival. • SOURCE trial (n = 1, 420), MRC. After nephrectomy, patients with high- and intermediate-risk RCC will be randomized to 3 years of sorafenib, 1 year of sorafenib and 2 years of placebo, or 3 years of placebo. The primary endpoint is metastases free survival.

Unaddressed Questions • Is any one agent better than the other? – Are they cross-resistant? – Are the studied doses/schedules optimal? • Can combination therapy improve outcome? • Can novel imaging modalities identify “benefiting” patients? • What is the role of these agents in the adjuvant setting? • What is the role of these agents in non-clear cell RCC?

What’s Hot in the Treatment of Renal Cell Carcinoma and is there Hope? • Oral VEGFR/PDGFR and m. TOR inhibitors are extremely active in clear cell RCC • They are much better tolerated than IFN or IL 2 but there is toxicity associated with these agents • These agents have changes how we treat this disease • Complete responses are extremely rare and the vast majority of patients eventually progress

What’s Hot in the Treatment of Renal Cell Carcinoma and is there Hope? • Strong rationale for targeting multiple pathways particularly angiogenesis in patients with advanced RCC • Novel signal transduction inhibitors have demonstrated an increase in PFS in 1 st and 2 nd line and an increase in survival in poor risk therapy naive patients • Some of these agents have been recently approved and others are still awaiting trial results • They are defining a new standard of care
 Res extra commercium
Res extra commercium Ira pré renal renal e pós renal
Ira pré renal renal e pós renal Renal cell carcinoma
Renal cell carcinoma Carcinoma renal de células claras fuhrman
Carcinoma renal de células claras fuhrman Cortical and juxtamedullary nephrons difference
Cortical and juxtamedullary nephrons difference Metastatic crc
Metastatic crc Borrmann classification of gastric cancer
Borrmann classification of gastric cancer Metastatic crc
Metastatic crc Kode icd 10 basal cell carcinoma
Kode icd 10 basal cell carcinoma Squamous cell carcinoma louisiana
Squamous cell carcinoma louisiana Basal cell carcinoma
Basal cell carcinoma Squamous cell carcinoma
Squamous cell carcinoma Lichen sclerosus vulvare
Lichen sclerosus vulvare Bcc pathology
Bcc pathology Hedgehog mites
Hedgehog mites Anaplastic squamous cell carcinoma
Anaplastic squamous cell carcinoma Whats hot whats not
Whats hot whats not Urinalysis
Urinalysis White hot vs red hot temperature
White hot vs red hot temperature Hot working and cold working
Hot working and cold working Perbedaan hot lava dan hot lava volcano
Perbedaan hot lava dan hot lava volcano Hot nor
Hot nor Slidetodoc.com
Slidetodoc.com Carcinoma in situ
Carcinoma in situ Neoplasia
Neoplasia Invasive ductal carcinoma with medullary features
Invasive ductal carcinoma with medullary features Manchester classification of breast cancer
Manchester classification of breast cancer Carcinoma de mama
Carcinoma de mama Carcinoma in situ
Carcinoma in situ Lobular breast
Lobular breast Wikipedia
Wikipedia Brown tumor
Brown tumor Breast papillary carcinoma
Breast papillary carcinoma Hormones
Hormones Carcinoma micropapilar invasivo de mama
Carcinoma micropapilar invasivo de mama Epithelial component
Epithelial component Carcinoma of stomach
Carcinoma of stomach Cancer de pulmon
Cancer de pulmon