Metamorphic Phase Diagrams Differ from Igneous Phase Diagrams


- Slides: 28

Metamorphic Phase Diagrams • Differ from Igneous Phase Diagrams • Show a snapshot of all compositions at given T, P • Rock remains at same point but diagram changes

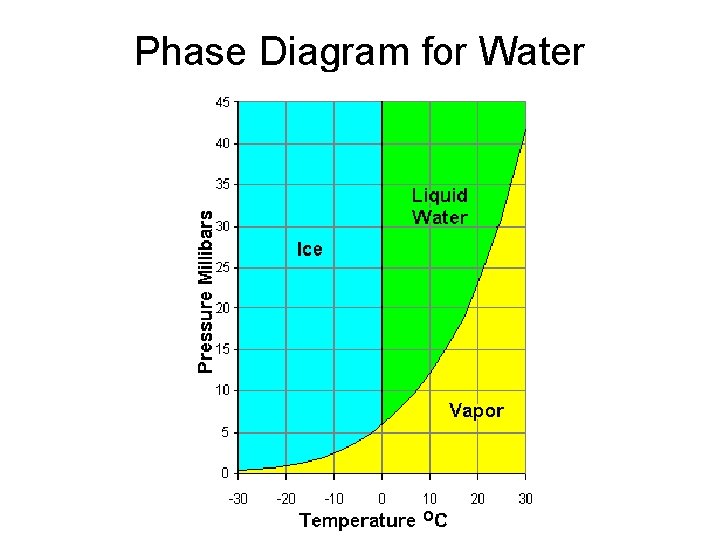
Phase Diagram for Water

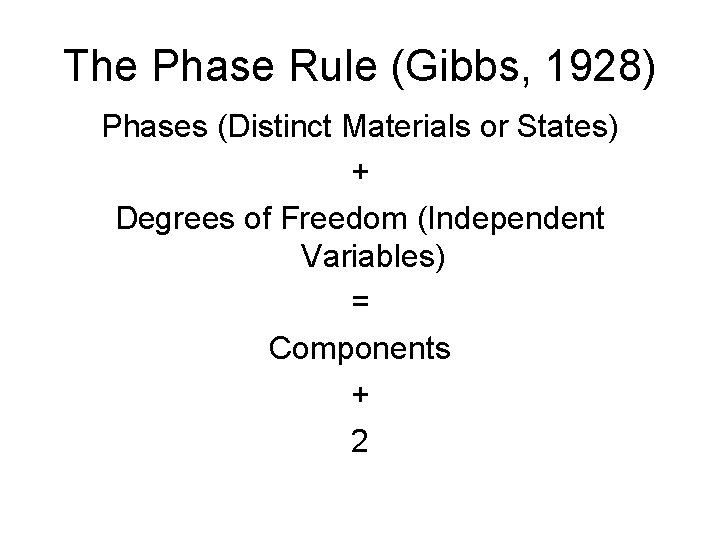
The Phase Rule (Gibbs, 1928) Phases (Distinct Materials or States) + Degrees of Freedom (Independent Variables) = Components + 2

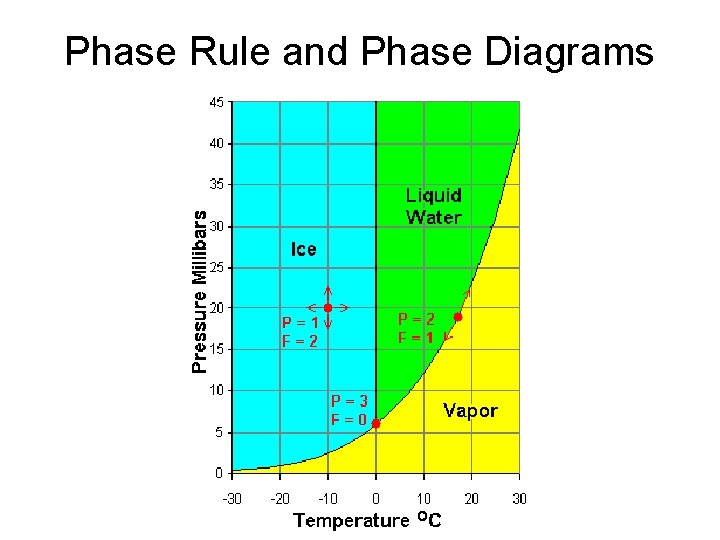
Phase Rule and Phase Diagrams

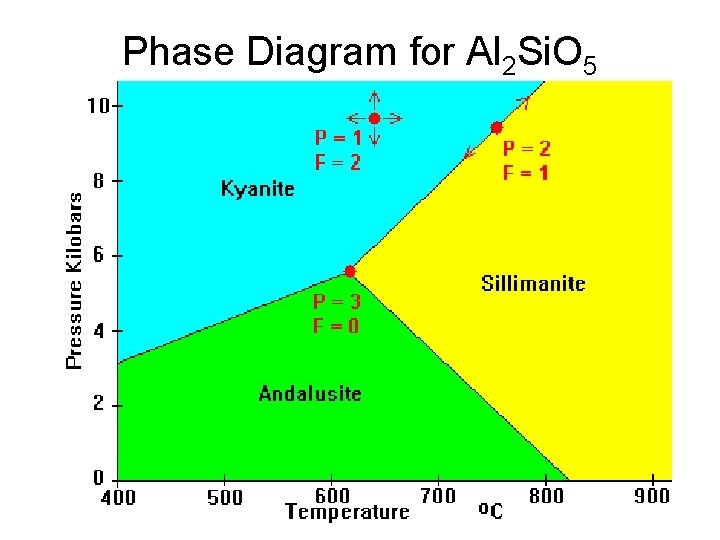
Phase Diagram for Al 2 Si. O 5

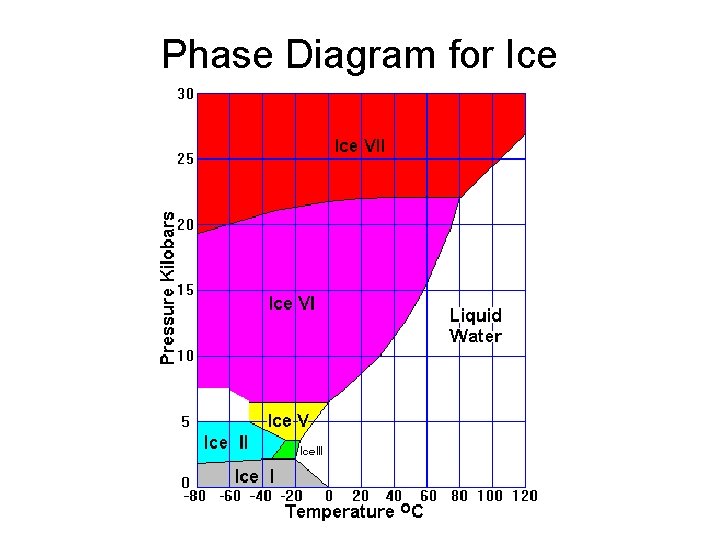
Phase Diagram for Ice

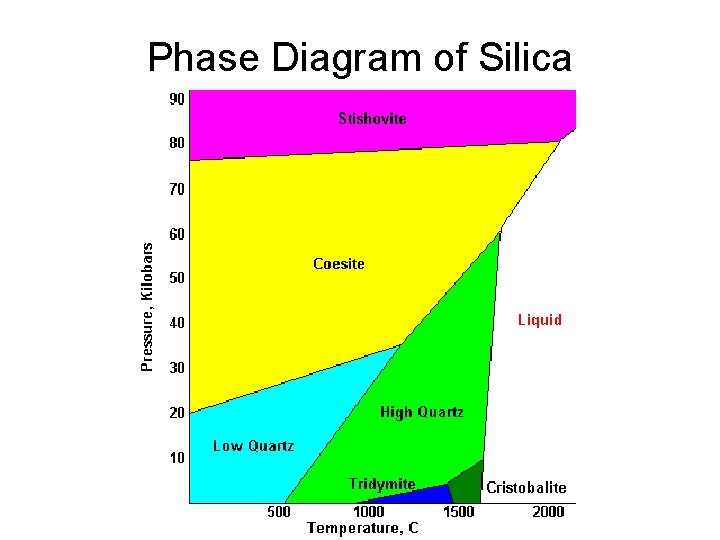
Phase Diagram of Silica

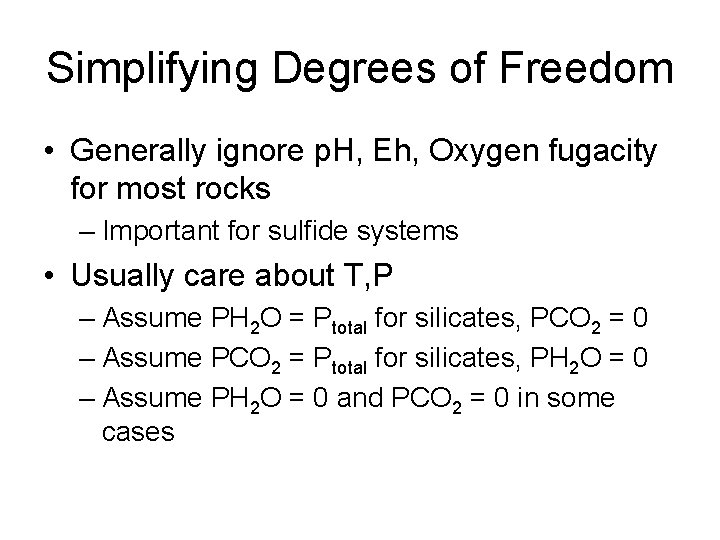
Degrees of Freedom • • Pressure Temperature PH 2 O PCO 2 p. H Oxygen fugacity Eh

Simplifying Degrees of Freedom • Generally ignore p. H, Eh, Oxygen fugacity for most rocks – Important for sulfide systems • Usually care about T, P – Assume PH 2 O = Ptotal for silicates, PCO 2 = 0 – Assume PCO 2 = Ptotal for silicates, PH 2 O = 0 – Assume PH 2 O = 0 and PCO 2 = 0 in some cases

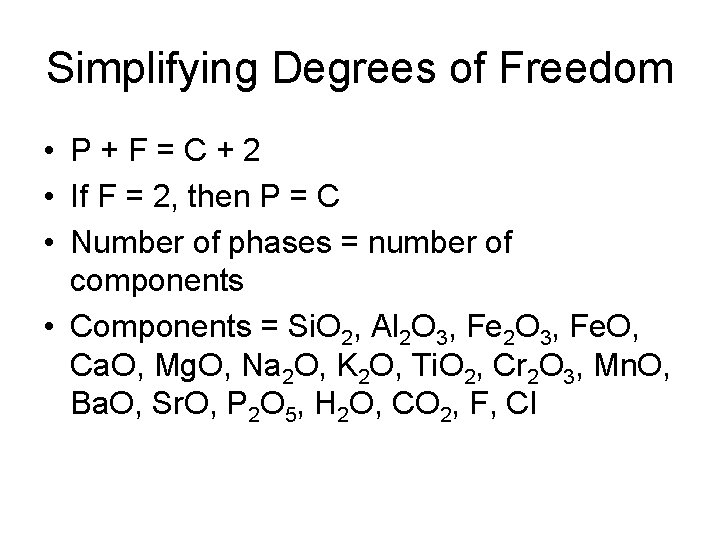
Simplifying Degrees of Freedom • P+F=C+2 • If F = 2, then P = C • Number of phases = number of components • Components = Si. O 2, Al 2 O 3, Fe. O, Ca. O, Mg. O, Na 2 O, K 2 O, Ti. O 2, Cr 2 O 3, Mn. O, Ba. O, Sr. O, P 2 O 5, H 2 O, CO 2, F, Cl

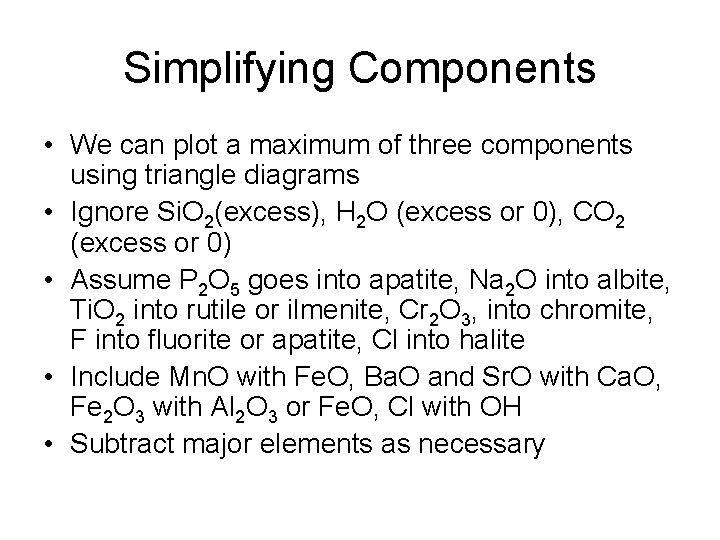
Simplifying Components • We can plot a maximum of three components using triangle diagrams • Ignore Si. O 2(excess), H 2 O (excess or 0), CO 2 (excess or 0) • Assume P 2 O 5 goes into apatite, Na 2 O into albite, Ti. O 2 into rutile or ilmenite, Cr 2 O 3, into chromite, F into fluorite or apatite, Cl into halite • Include Mn. O with Fe. O, Ba. O and Sr. O with Ca. O, Fe 2 O 3 with Al 2 O 3 or Fe. O, Cl with OH • Subtract major elements as necessary

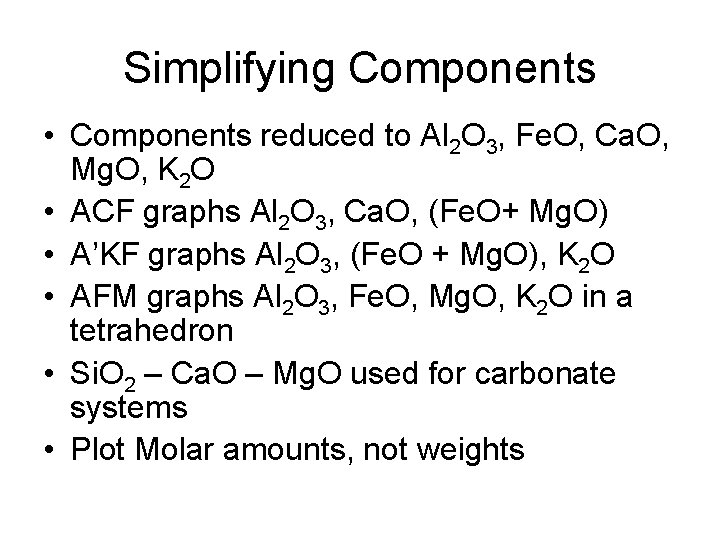
Simplifying Components • Components reduced to Al 2 O 3, Fe. O, Ca. O, Mg. O, K 2 O • ACF graphs Al 2 O 3, Ca. O, (Fe. O+ Mg. O) • A’KF graphs Al 2 O 3, (Fe. O + Mg. O), K 2 O • AFM graphs Al 2 O 3, Fe. O, Mg. O, K 2 O in a tetrahedron • Si. O 2 – Ca. O – Mg. O used for carbonate systems • Plot Molar amounts, not weights

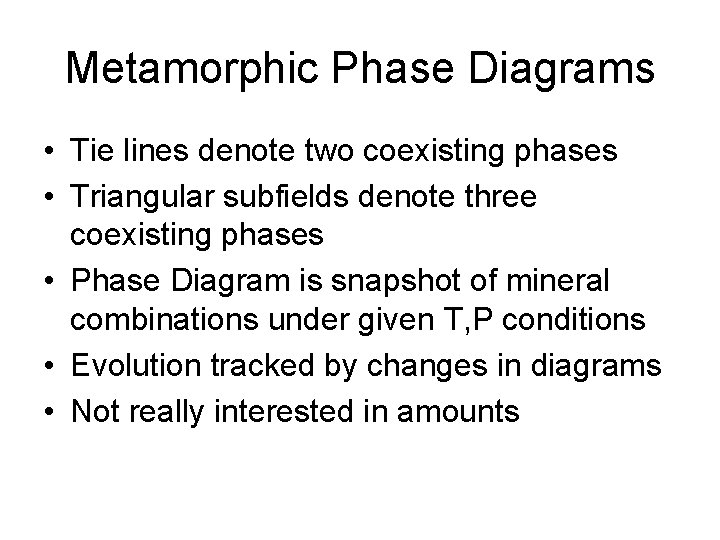
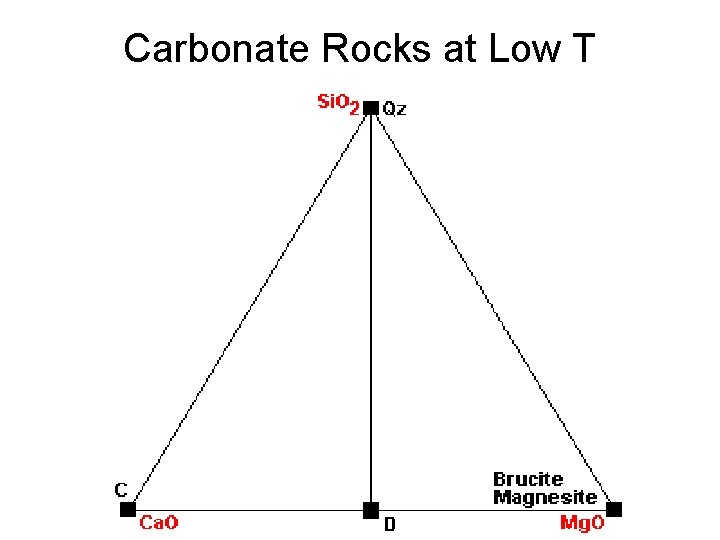
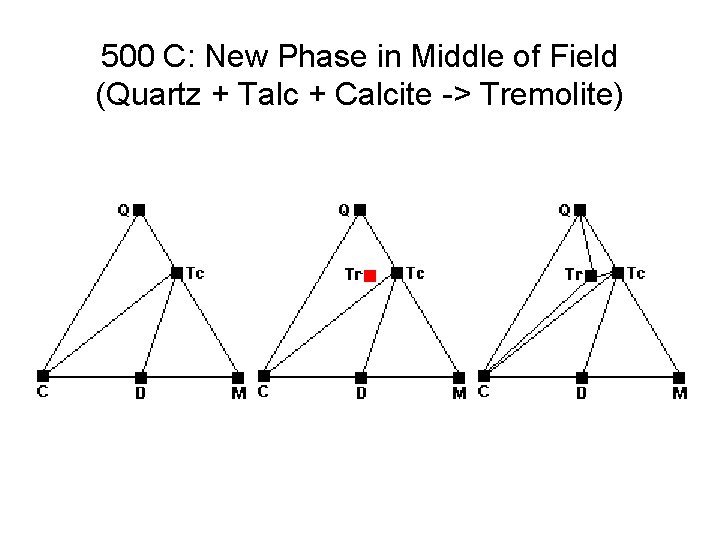
Metamorphic Phase Diagrams • Tie lines denote two coexisting phases • Triangular subfields denote three coexisting phases • Phase Diagram is snapshot of mineral combinations under given T, P conditions • Evolution tracked by changes in diagrams • Not really interested in amounts

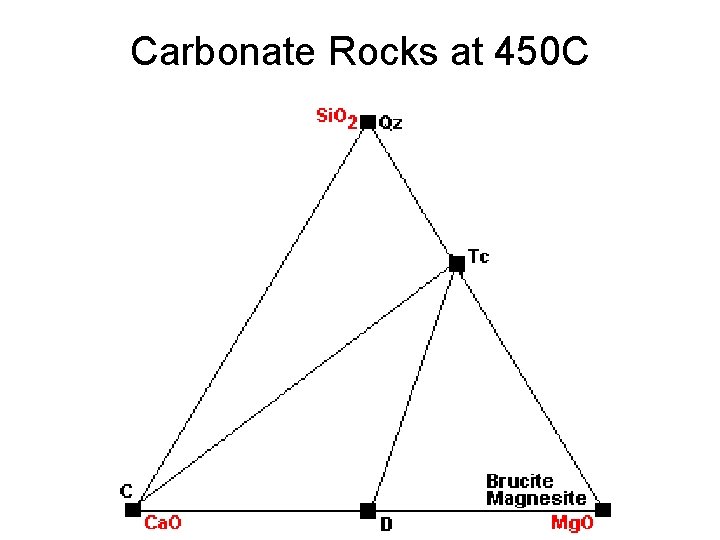
Carbonate Rocks at 450 C

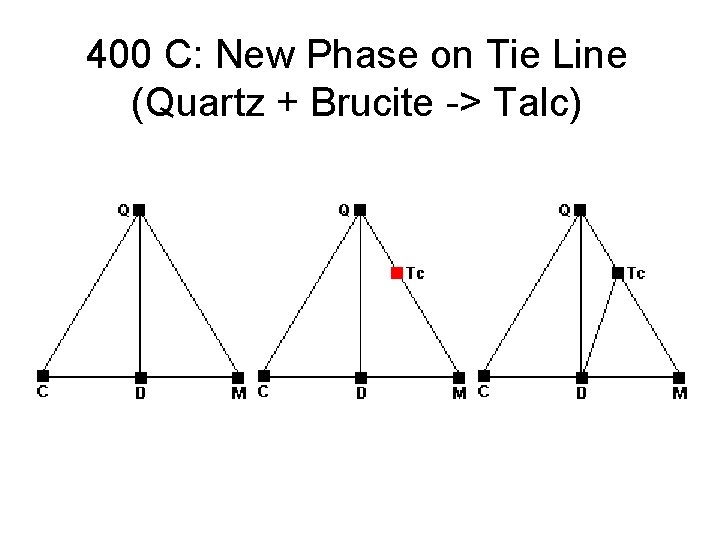
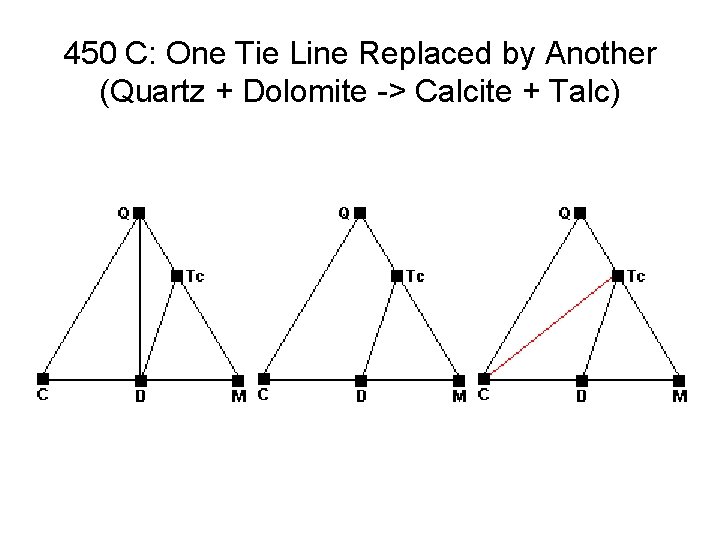
Metamorphic Phase Diagrams • Normally F = 2, C = 3, P = 3 • On a reaction curve, F = 1, P = 4 • How to get 4 Phases Together: – New Phase Appears in Middle of Field – New Phase Appears on Tie Line – Tie Line Breaks and New One Forms • Changes in Metamorphism – New Minerals Appear – Old Minerals Disappear – Compatibilities Shift

Carbonate Rocks at Low T

400 C: New Phase on Tie Line (Quartz + Brucite -> Talc)

450 C: One Tie Line Replaced by Another (Quartz + Dolomite -> Calcite + Talc)

500 C: New Phase in Middle of Field (Quartz + Talc + Calcite -> Tremolite)

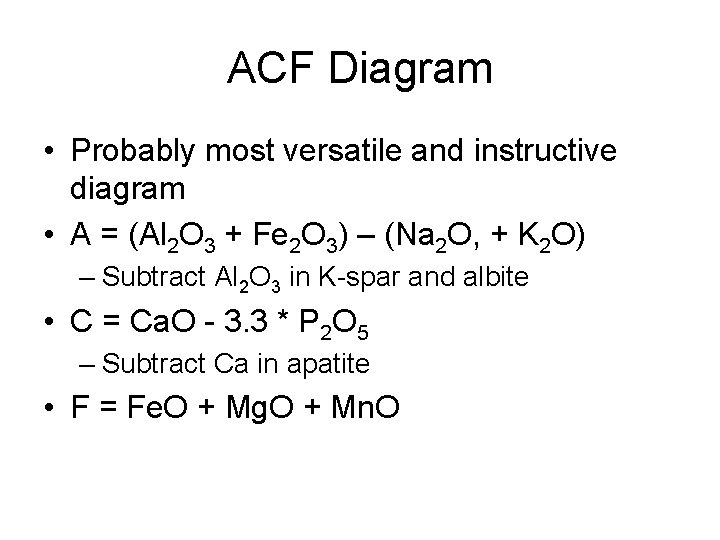
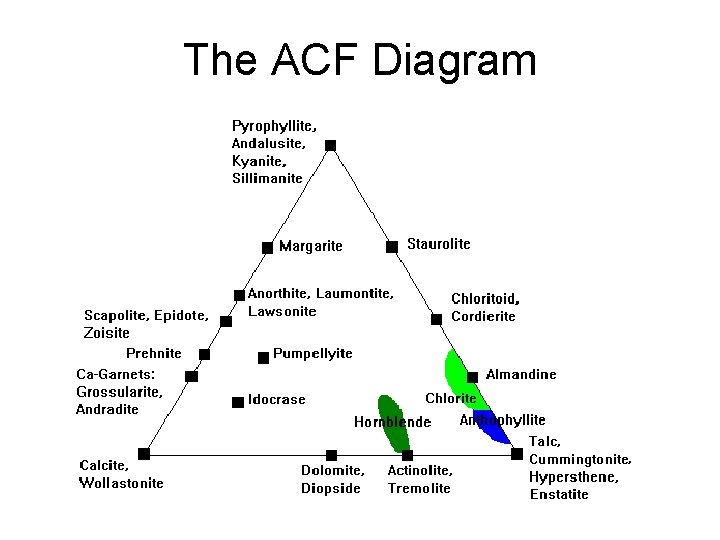
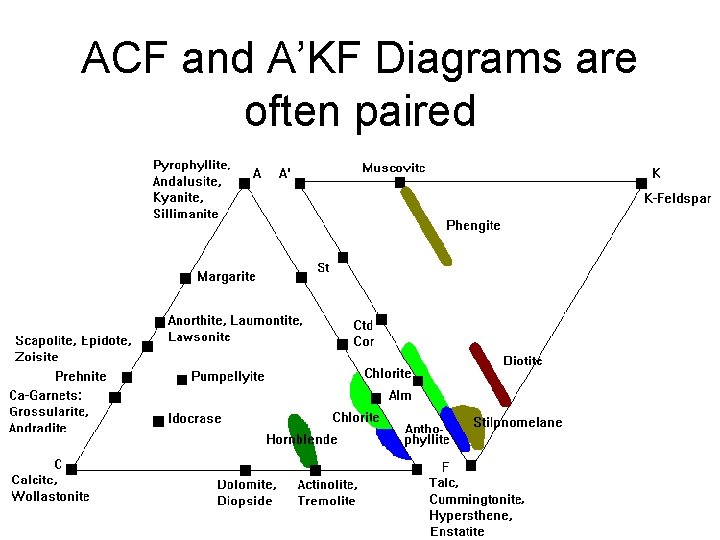
ACF Diagram • Probably most versatile and instructive diagram • A = (Al 2 O 3 + Fe 2 O 3) – (Na 2 O, + K 2 O) – Subtract Al 2 O 3 in K-spar and albite • C = Ca. O - 3. 3 * P 2 O 5 – Subtract Ca in apatite • F = Fe. O + Mg. O + Mn. O

The ACF Diagram

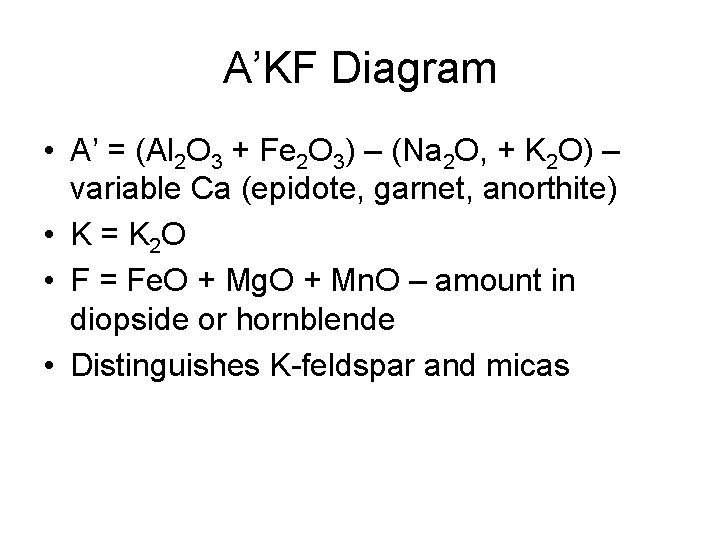
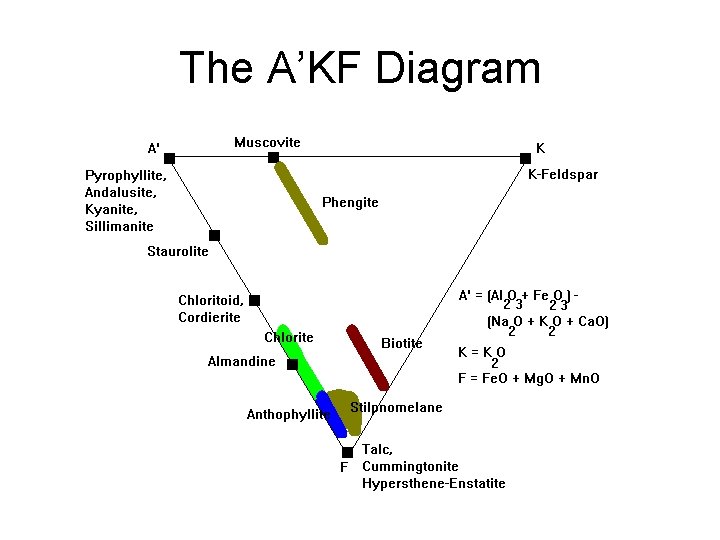
A’KF Diagram • A’ = (Al 2 O 3 + Fe 2 O 3) – (Na 2 O, + K 2 O) – variable Ca (epidote, garnet, anorthite) • K = K 2 O • F = Fe. O + Mg. O + Mn. O – amount in diopside or hornblende • Distinguishes K-feldspar and micas

The A’KF Diagram

ACF and A’KF Diagrams are often paired

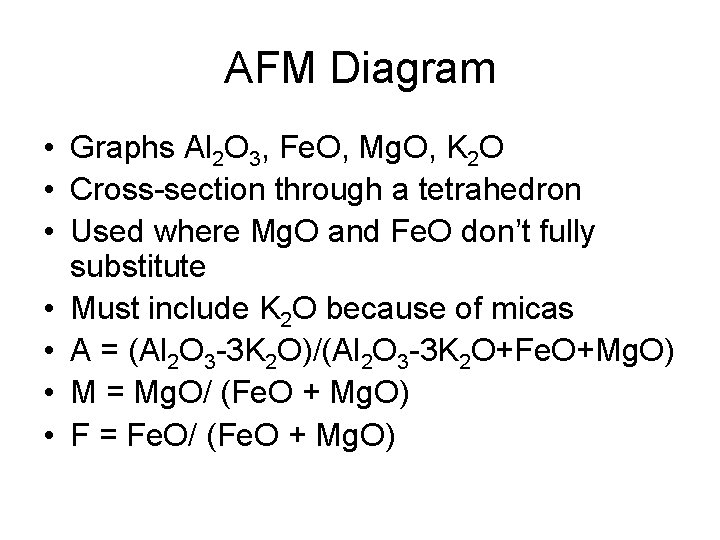
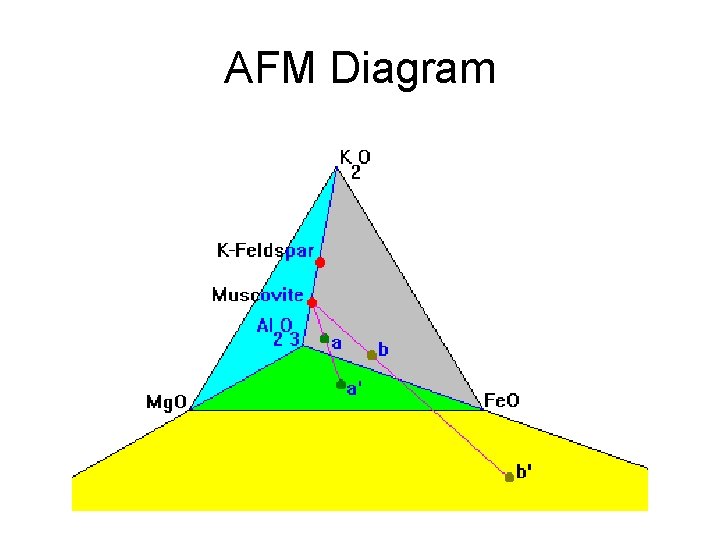
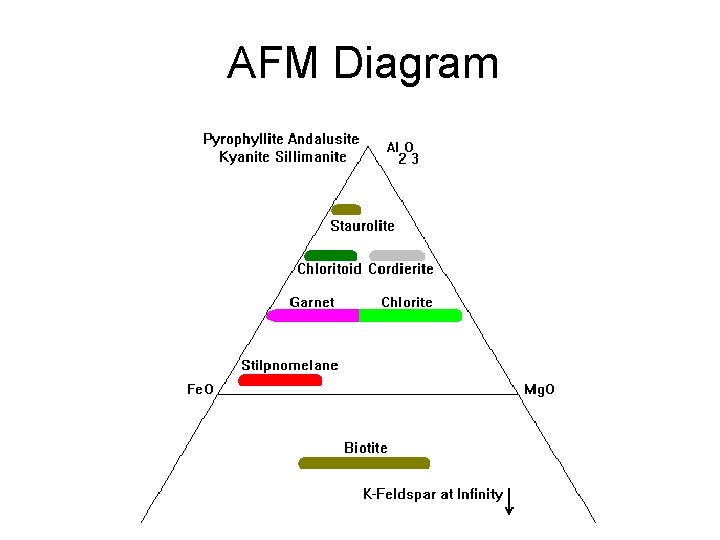
AFM Diagram • Graphs Al 2 O 3, Fe. O, Mg. O, K 2 O • Cross-section through a tetrahedron • Used where Mg. O and Fe. O don’t fully substitute • Must include K 2 O because of micas • A = (Al 2 O 3 -3 K 2 O)/(Al 2 O 3 -3 K 2 O+Fe. O+Mg. O) • M = Mg. O/ (Fe. O + Mg. O) • F = Fe. O/ (Fe. O + Mg. O)

AFM Diagram

AFM Diagram

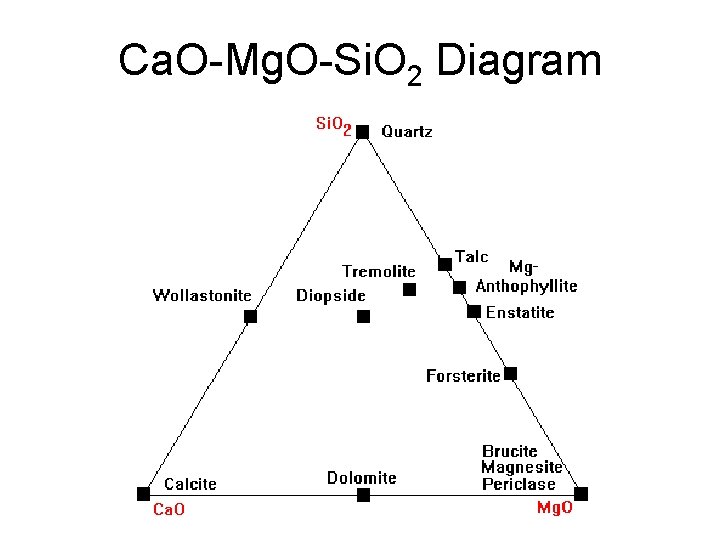
Ca. O-Mg. O-Si. O 2 Diagram