Metals Starter Activity Describe three processes for extracting

Metals

Starter Activity • Describe three processes for extracting metals. • Which process is for the most reactive metals? • Name 2 alkali metals • Name 2 transition metals • Name 2 alkaline earth metals • Explain why aluminium doesn‘t corrode.

Alkali Metals Properties • • • All very reactive and have to be stored under oil. Reactivity increases as you move down the group. They are soft and can be cut with a knife. React violently with water. http: //www. youtube. com/watch? v=m 55 kgy. Ap. Yr Y Metal + water Sodium + water metal hydroxide + hydrogen sodium hydroxide + hydrogen

Flame Tests – Alkali Metals • Metal compounds, especially those of the alkali metals, can be identified by a flame test. • http: //www. youtube. com/watch? v=MGUPKA _p. OEE

Compounds of Alkali Metals • Alkali metals are not very useful…. . why? • Compounds of alkali metals are very useful: – Na. Cl – salt – Na. NO 2 and KNO 2 – fertilizers and explosives – Na 2 CO 3 – washing powder – Na. HCO 3 – baking soda – Na. OH – oven cleaner
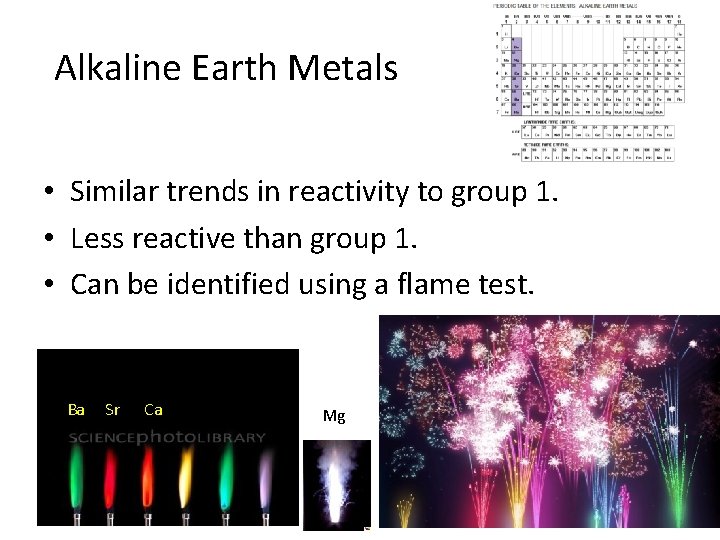
Alkaline Earth Metals • Similar trends in reactivity to group 1. • Less reactive than group 1. • Can be identified using a flame test. Ba Sr Ca Mg

Activity • Quick check questions – page 234 (5 minutes)
Transition Metals • Most useful metal elements. • All have typical metals properties: – – – • • Hard and strong High melting points High density Good heat and electrical conductors Are malleable and ductile Coloured compounds Can form more than one type of ion. Make useful catalysts Some are magnetic.

Transition metals have coloured compounds • Salts are generally white solids……but…. . if you dissolve them in water they produce coloured solutions (chart on page 237 in text book).

Variable Valencies • Group 1 metals always form 1+ ions • Group 2 metals always form 2+ ions • Group 3 (Aluminium) always forms 3+ ions • Transition metals are not so straight forward. • Iron (Fe) can form 2+ or 3+ ions. – Fe O (green) – Fe 2 O 3 (red brown)

Test for Iron Ions • Add an alkali solution; – Sodium hydroxide (Na. OH) – Ammonia solution • Iron (II) salts will produce a green jelly like precipitate Fe. Cl 2 + 2 Na. OH Fe(OH)2 + 2 Na. Cl • Iron (III) salts will produce a red-brown precipitate. Fe. Cl 3 + 3 Na. OH Fe(OH)3 + 3 Na. Cl
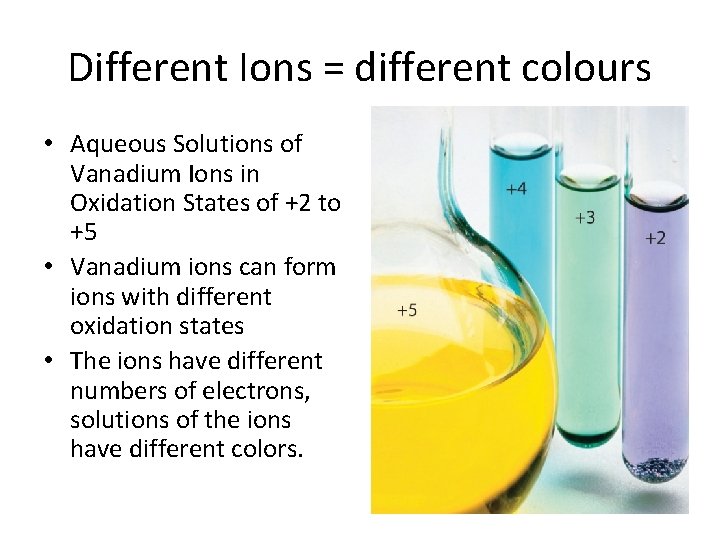
Different Ions = different colours • Aqueous Solutions of Vanadium Ions in Oxidation States of +2 to +5 • Vanadium ions can form ions with different oxidation states • The ions have different numbers of electrons, solutions of the ions have different colors.

Homework - Aluminium • Write a paragraph on the usefulness of aluminium. • Describe the „Thermit“ reaction. • Describe the analytical test for Aluminium.
- Slides: 13