Metals Reactivity of metals 1 Reactivity of Metals







- Slides: 16

Metals Reactivity of metals 1

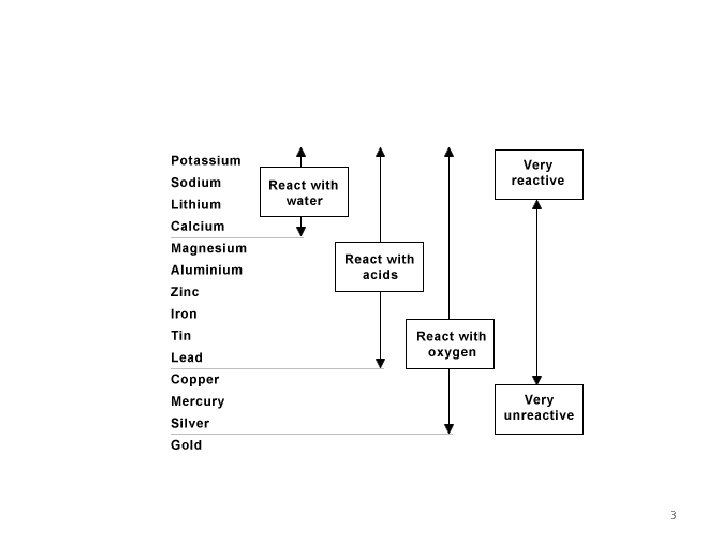
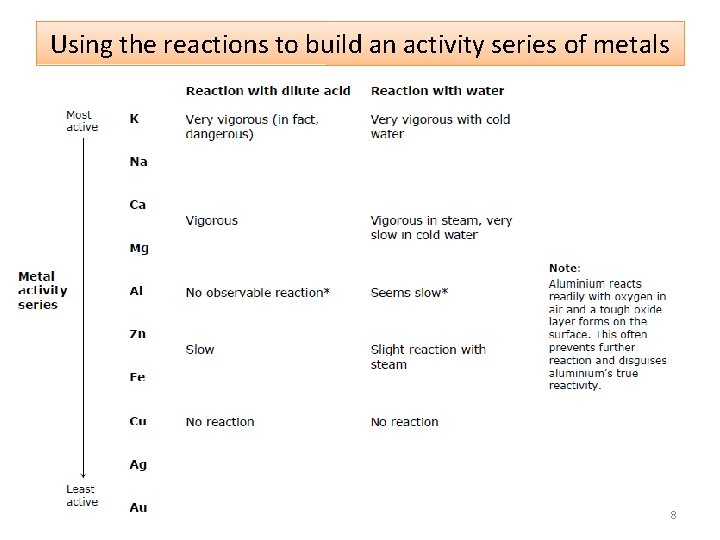
Reactivity of Metals • Some substances are more reactive than others, and this can be observed in reactions involving metals. • Metals undergo oxidation reactions where they lose electrons. The activity series lists metals in order of their reactivity; their ability to lose electrons. • Reactivity can be observed by a metal’s reaction with water and dilute acid to produce H 2 gas. • In a displacement reaction a more active metal loses its electrons to a less active metal. 2

3

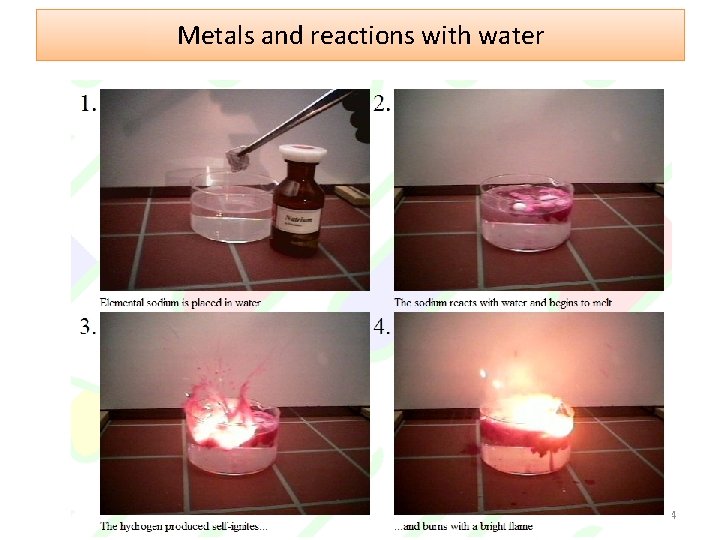
Metals and reactions with water Group 1 metals react violently with water 4

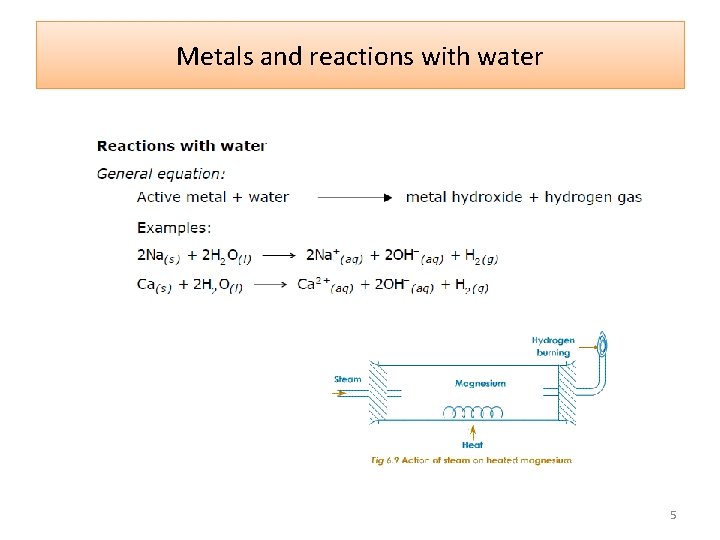
Metals and reactions with water 5

Metals and reactions with dilute acids Group 1 metals are considered too dangerous to react with dilute acid This is their reaction with air and with water: https: //www. youtube. com/watch? v=uixx. Jt. JPVXk Ca Mg Zn Fe 6

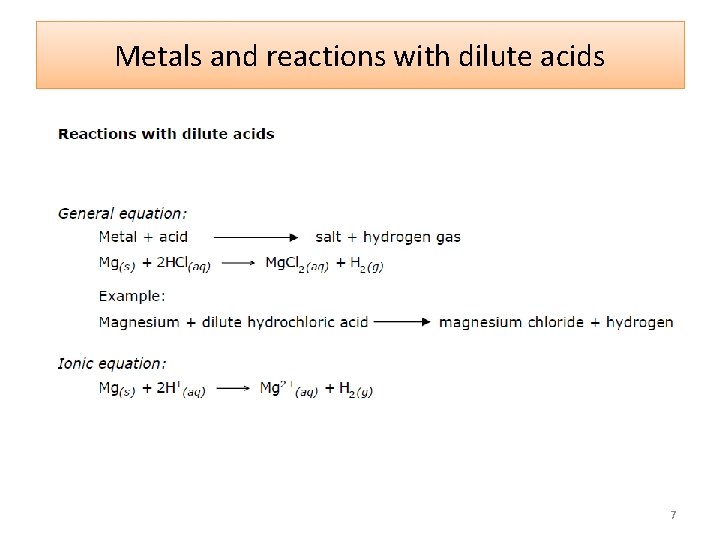
Metals and reactions with dilute acids 7

Using the reactions to build an activity series of metals 8

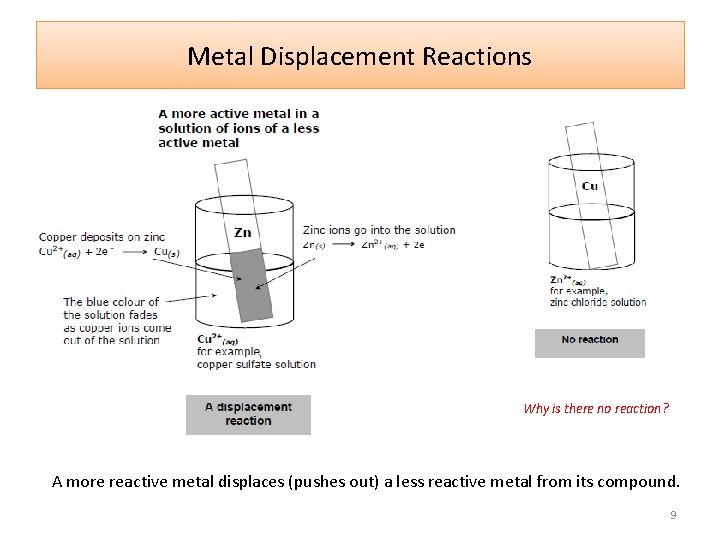
Metal Displacement Reactions Why is there no reaction? A more reactive metal displaces (pushes out) a less reactive metal from its compound. 9

Problems • Write equations for the reactions of: – Potassium and water – Zinc and water • Describe and compare how the two metals react with water 10

Problems • Write an ionic equation for the reaction of zinc with dilute sulphuric acid • Write the two half equations for the reaction and identify which half equation is oxidation and which is reduction 11

Problems • Write half equations and a complete ionic equation for the displacement reaction that occurs when magnesium metal is added to a solution of zinc ions • Predict whether displacement reaction occur when the following metals and ions are combined. Write half equations and complete equations for any reactions that occur – Zn(s) and Cu 2+(aq) – Cu(s) and Fe 2+(aq) – Mg(s) and Cu 2+(aq) 12

The Essentials • Pg. 125 -127 13

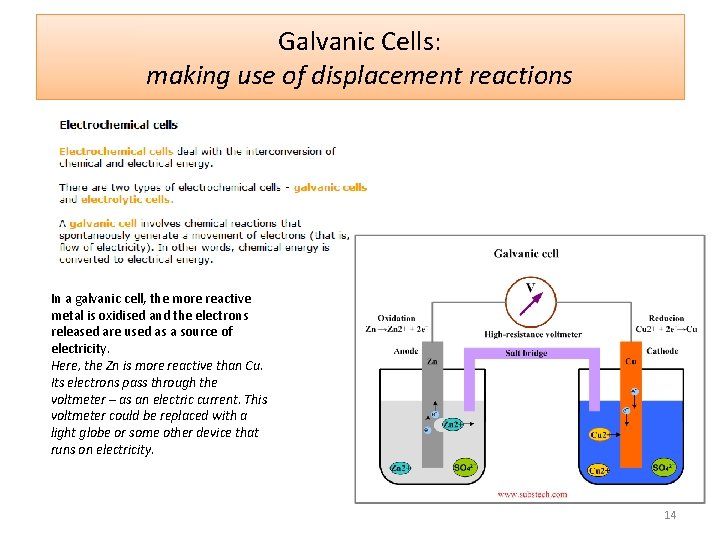
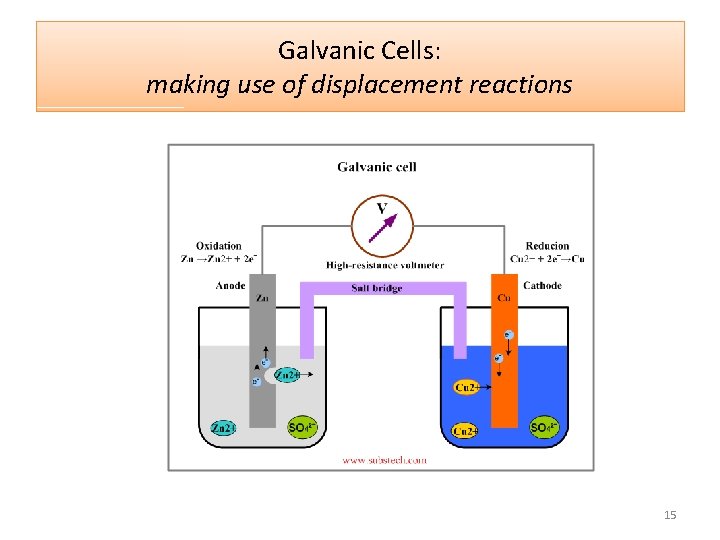
Galvanic Cells: making use of displacement reactions In a galvanic cell, the more reactive metal is oxidised and the electrons released are used as a source of electricity. Here, the Zn is more reactive than Cu. Its electrons pass through the voltmeter – as an electric current. This voltmeter could be replaced with a light globe or some other device that runs on electricity. 14

Galvanic Cells: making use of displacement reactions 15

The Essentials • Pg. 128 -136 16