Metals Of the known naturally occurring elements the














- Slides: 16

Metals Of the known naturally occurring elements, the major proportion are metals. The most common are: Aluminium 8% Iron 5% Metals are divided into two general categories:

Metal Ferrous Metals These are the group of metals whose main composition is IRON. The majority of ferrous metals are magnetic. Examples: Cast Iron Mild Steel Stainless Steel Medium and High Carbon Steels

Metal Non Ferrous Metals This group of metals are those which DO NOT contain iron and include: Aluminium Copper Gold Silver Tin Lead

Metal Alloys Alloying metals is carried out to change the physical properties of a metal i. e. increase tensile strength, malleability, corrosion resistance and ductility etc. An alloy is a metal formed by mixing together different metals or by adding other elements.

Metal Alloys Examples: • Steel = Iron + …………. . • ………………. = Copper + Zinc • Duraluminium = Aluminium + ………………. . • ………… = Iron + Carbon + Chromium • Bronze = …………. . + Tin

Metal Heat Treatment Video: The Heat Treatment Process Video: Copper Annealing Another way of producing desirable properties in metals is to chang it’s properties through heat. These processes include; ANNEALING = softening a metal to make it easier to form TEMPERING = Increasing the toughness of a metal by reducing it's hardness HARDENING = Increasing the metal's hardness and increase it's resistance to wear

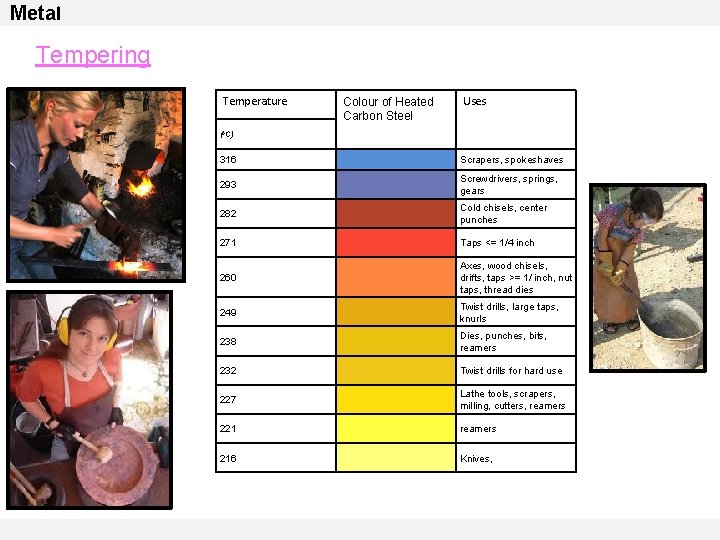
Metal Tempering Temperature Colour of Heated Carbon Steel Uses (o. C) 316 Scrapers, spokeshaves 293 Screwdrivers, springs, gears 282 Cold chisels, center punches 271 Taps <= 1/4 inch 260 Axes, wood chisels, drifts, taps >= 1/ inch, nut taps, thread dies 249 Twist drills, large taps, knurls 238 Dies, punches, bits, reamers 232 Twist drills for hard use 227 Lathe tools, scrapers, milling, cutters, reamers 221 reamers 216 Knives,

Metal Joining metals There are 3 ways to join metals – Mechanical, heat and chemical Mechanical Joints These joints include: • Riveting • Nuts and bolts • Self Tapping screws • Self securing joints Video: Hot Riveting

Metal Heat Joints Heat can be used to join the metal together and include: Soldering and brazing. This form of joining involves the following stages: • • • Clean metal Add flux (a chemical used to keep the metal clean) Heat to required temperature Add spelter (brazing rod) Allow to cool

Metal Heat Joints Welding: This form of joining involves melting the two “parent” materials together and is therefore at a much higher temperature, i. e. ………. o. C for steel. • • Examples of welding include: Oxyacetylene M. I. G. and T. I. G. Electric stick welding

Metal Joining metals – chemical These are glued joints. With metal the common adhesive is called ………………. .

Metal Casting Video: Aluminium Casting

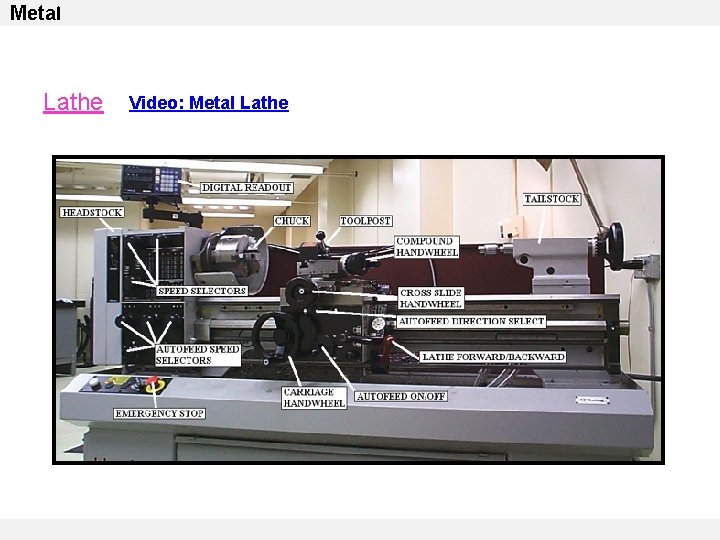
Metal Lathe Video: Metal Lathe Video

Metal Milling Video: Milling Machine Video

Metal Machining Video: CNC Metal Lathe Video: CNC Metal Milling

Metal Finishes are applied to some metals to: Protect them from corrosion Improve appearance Video: Powder Coating Video: Electroplating EXAMPLES ……………………………………………….