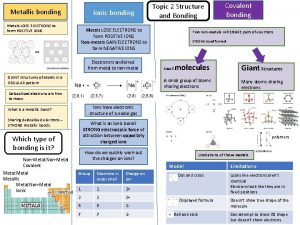
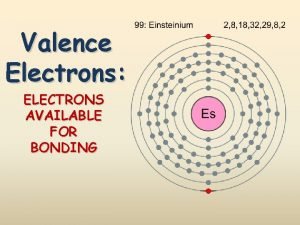
metals lose valence electrons form cation ion nonmetals










![Lewis Diagram of calcium iodide (Ca. I 2) ·· -1 +2 [Ca] [: I: Lewis Diagram of calcium iodide (Ca. I 2) ·· -1 +2 [Ca] [: I:](https://slidetodoc.com/presentation_image_h2/3e2d142d3933d715b3b794c4612990be/image-17.jpg)

- Slides: 19


• metals: lose valence electrons – form cation (+ ion) • non-metals: gain electrons – form anion (- ion)

Ionic Bond • occurs between: metal element & non-metal element • involves transfer of electrons between metal & non-metal elements to form ions • # electrons lost by cation(s) = # electrons gained by anion(s)

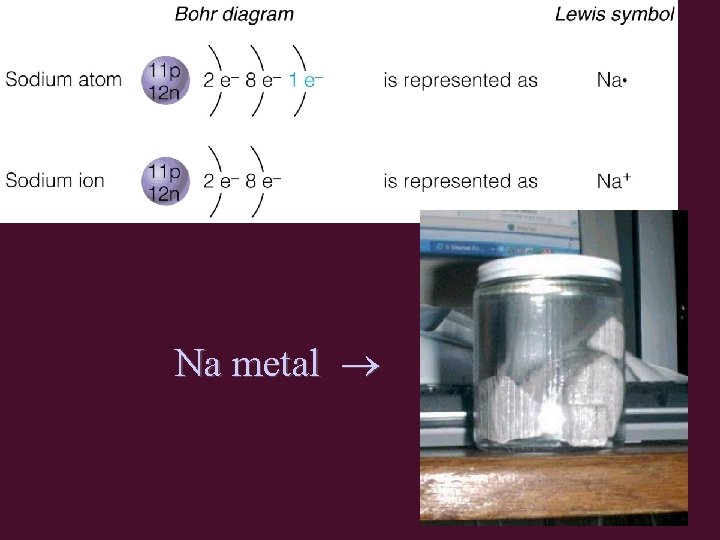
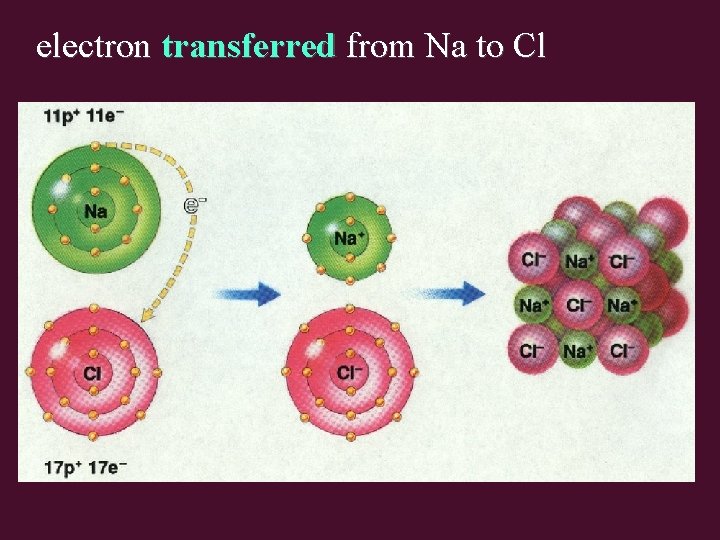
Metals are Losers! • Na atom configuration: 2 -8 -1 • Na loses 1 electron → Na+1 ion – Na+1 configuration is 2 -8 • same as Ne configuration (2 -8)

Na metal

elements from what group _? _ will easily take the one valence electron from Na? ? what group has 7 valence electrons so only need one more? group 1 elements will easily give up their one valence electron to the elements in group 17

Non-metals are Winners! • Cl configuration: 2 -8 -7 • Cl gains 1 electron → Cl-1 ion – Cl-1 has the configuration 2 -8 -8 • same as Ar configuration (2 -8 -8)

Cl 2 (g)

electron transferred from Na to Cl


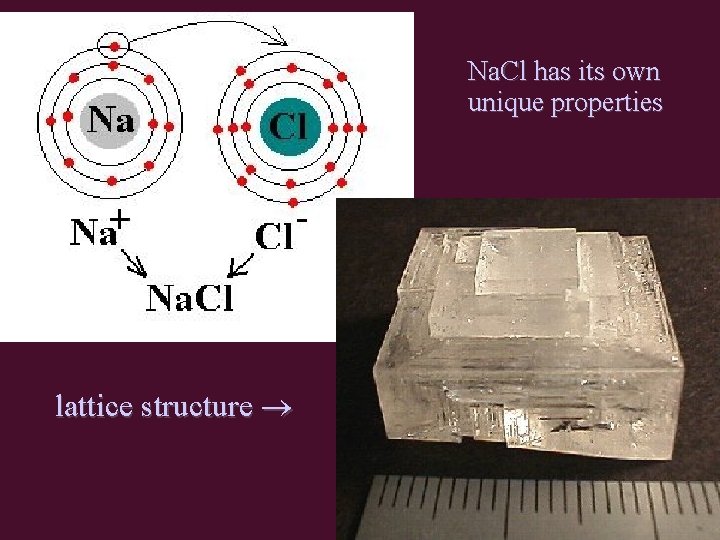
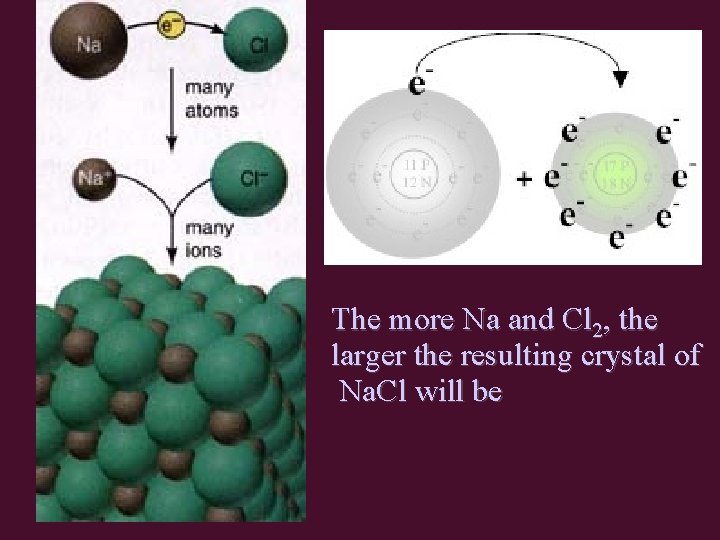
Structure of Ionic Compounds • oppositely charged ions are attracted to each other by strong electrostatic interactions (+/-) • (+/-) ions form crystal lattice – regular 3 -D pattern or array – ions held in fixed positions (solid state) • Unit Cell = smallest repetitive unit in lattice

Na. Cl has its own unique properties lattice structure

The more Na and Cl 2, the larger the resulting crystal of Na. Cl will be

different representations of a crystal lattice

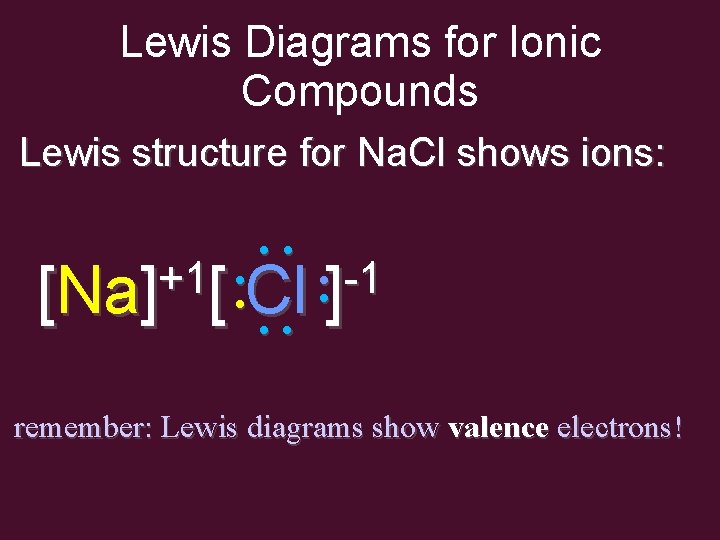
Lewis Diagrams for Ionic Compounds Lewis structure for Na. Cl shows ions: • • • • -1 +1 [Na] [ • Cl • ] • • remember: Lewis diagrams show valence electrons!

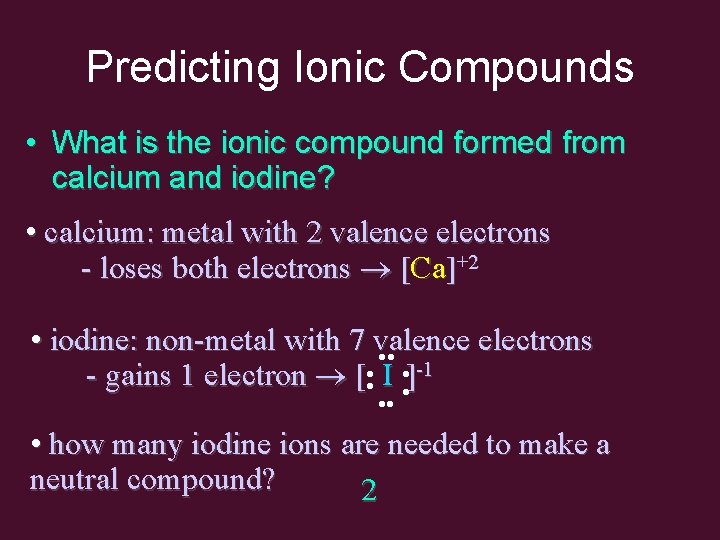
Predicting Ionic Compounds • What is the ionic compound formed from calcium and iodine? • calcium: metal with 2 valence electrons - loses both electrons [Ca]+2 • iodine: non-metal with 7 valence electrons • • -1 - gains 1 electron [ • • I • • ] • • • how many iodine ions are needed to make a neutral compound? 2
![Lewis Diagram of calcium iodide Ca I 2 1 2 Ca I Lewis Diagram of calcium iodide (Ca. I 2) ·· -1 +2 [Ca] [: I:](https://slidetodoc.com/presentation_image_h2/3e2d142d3933d715b3b794c4612990be/image-17.jpg)
Lewis Diagram of calcium iodide (Ca. I 2) ·· -1 +2 [Ca] [: I: ] ·· ·· or ·· -1 +2 [: I: ] [Ca] [: I: ] ·· ·· note: total charge MUST add up to zero since compounds are neutral

Properties of Ionic Compounds • • • high melting points low vapor pressures tend to be hard and brittle solids do not conduct electricity molten (liquid) states do conduct electricity aqueous solutions do conduct electricity

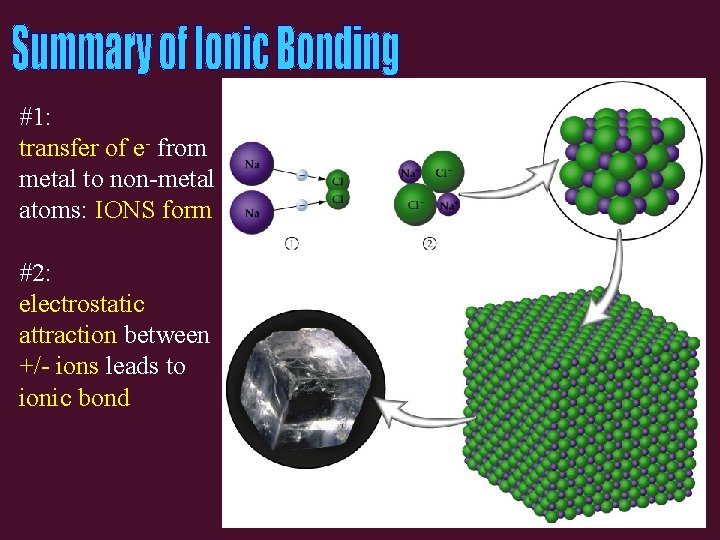
#1: transfer of e- from metal to non-metal atoms: IONS form #2: electrostatic attraction between +/- ions leads to ionic bond
 Metal lose electrons to form
Metal lose electrons to form Habit 4: think win-win examples
Habit 4: think win-win examples Win win win lose lose lose
Win win win lose lose lose Win win situacija
Win win situacija Types of bonds between atoms
Types of bonds between atoms Metals lose electrons
Metals lose electrons Group 13 elements
Group 13 elements Transition metals valence electrons
Transition metals valence electrons The octet rule states that
The octet rule states that Sulfite ion valence electrons
Sulfite ion valence electrons Cl2co lewis structure
Cl2co lewis structure Metals react with nonmetals to form ionic compounds by
Metals react with nonmetals to form ionic compounds by It is hardest for me to think win-win when
It is hardest for me to think win-win when Example of a win win situation
Example of a win win situation Metals vs nonmetals
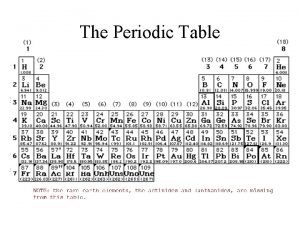
Metals vs nonmetals Periodic table metals nonmetals metalloids noble gases
Periodic table metals nonmetals metalloids noble gases Periodic table metals nonmetals semimetals
Periodic table metals nonmetals semimetals Metal vs non metal
Metal vs non metal Least reactive non-metal
Least reactive non-metal Metals metalloids and nonmetals periodic table
Metals metalloids and nonmetals periodic table