METALS Lead Pb Unique properties used since antiquity

METALS

Lead, Pb • Unique properties - used since antiquity • Mostly anthropogenic sources (Greenland snow pack data, 1954) • Banned as paint additive – Europe 1921 – USA 1978 • Sources: smelters, refineries, power plants, incinerators, manufacturing and recycling operations
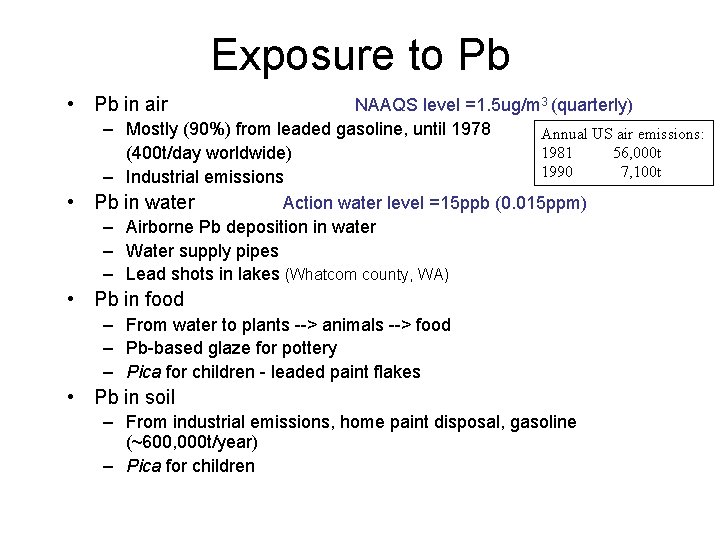
Exposure to Pb • Pb in air – – • Pb – – – NAAQS level =1. 5 ug/m 3 (quarterly) Mostly (90%) from leaded gasoline, until 1978 Annual US air emissions: 1981 56, 000 t (400 t/day worldwide) 1990 7, 100 t Industrial emissions in water Action water level =15 ppb (0. 015 ppm) Airborne Pb deposition in water Water supply pipes Lead shots in lakes (Whatcom county, WA) • Pb in food – From water to plants --> animals --> food – Pb-based glaze for pottery – Pica for children - leaded paint flakes • Pb in soil – From industrial emissions, home paint disposal, gasoline (~600, 000 t/year) – Pica for children

Pb Health effects • Young animals and humans more susceptible • Aquatic organisms and birds affected (directly or by water acidification)
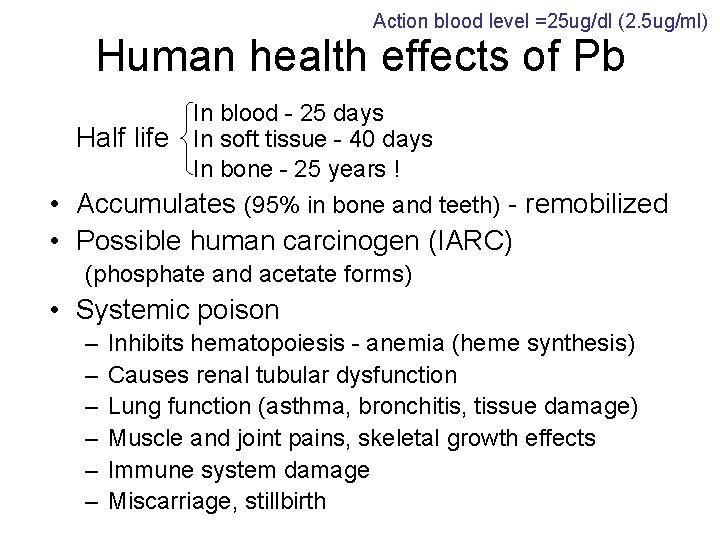
Action blood level =25 ug/dl (2. 5 ug/ml) Human health effects of Pb In blood - 25 days Half life In soft tissue - 40 days In bone - 25 years ! • Accumulates (95% in bone and teeth) - remobilized • Possible human carcinogen (IARC) (phosphate and acetate forms) • Systemic poison – – – Inhibits hematopoiesis - anemia (heme synthesis) Causes renal tubular dysfunction Lung function (asthma, bronchitis, tissue damage) Muscle and joint pains, skeletal growth effects Immune system damage Miscarriage, stillbirth

Children more vulnerable CNS effects from blood level =10 ug/dl (1 ug/ml) - CDC • Pb poisoning is the most common and serious environmental disease • Primary target CNS – Retardation and brain damage – Behavioral changes – Cognitive development • Levels dropped since 1974 from 15 -18 to 23 ug/dl - still 2. 2% US children are above the 10 ug/dl limit
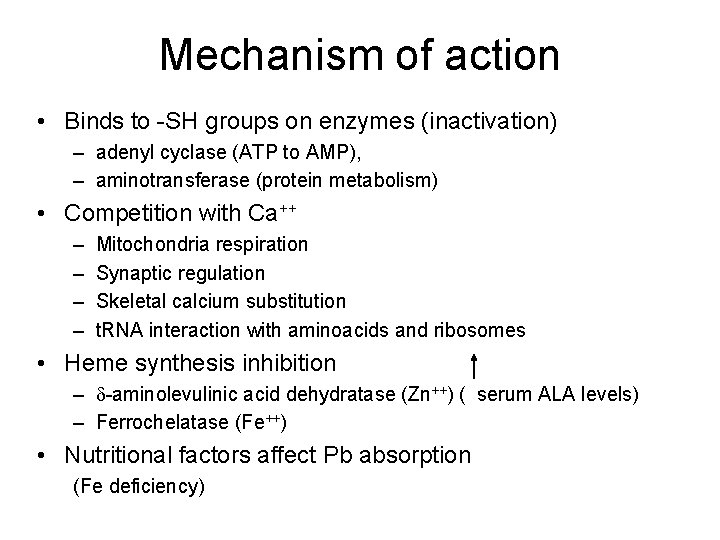
Mechanism of action • Binds to -SH groups on enzymes (inactivation) – adenyl cyclase (ATP to AMP), – aminotransferase (protein metabolism) • Competition with Ca++ – – Mitochondria respiration Synaptic regulation Skeletal calcium substitution t. RNA interaction with aminoacids and ribosomes • Heme synthesis inhibition – -aminolevulinic acid dehydratase (Zn++) ( serum ALA levels) – Ferrochelatase (Fe++) • Nutritional factors affect Pb absorption (Fe deficiency)
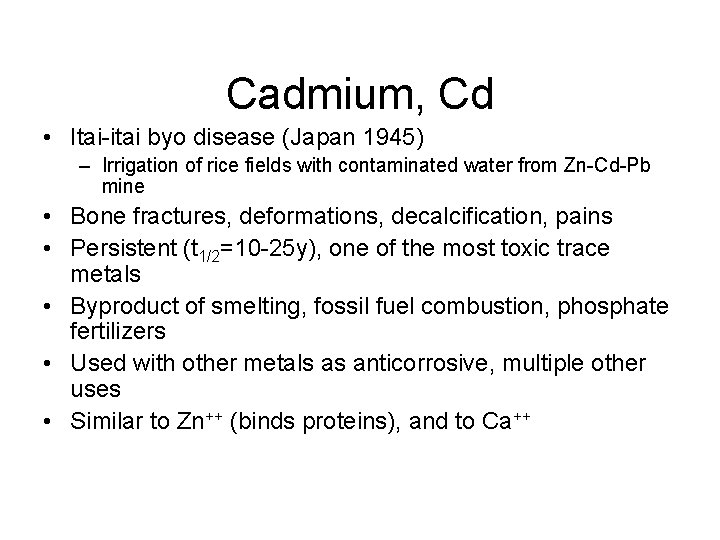
Cadmium, Cd • Itai-itai byo disease (Japan 1945) – Irrigation of rice fields with contaminated water from Zn-Cd-Pb mine • Bone fractures, deformations, decalcification, pains • Persistent (t 1/2=10 -25 y), one of the most toxic trace metals • Byproduct of smelting, fossil fuel combustion, phosphate fertilizers • Used with other metals as anticorrosive, multiple other uses • Similar to Zn++ (binds proteins), and to Ca++
Exposure to Cd - t 1/2 = 7. 4 -18 y • Air - 25 -40% retention OSHA air 200 ug/m 3 – Mostly occupational – Ambient air 1 ng/m 3 (20 -50 ng/day) – Tobacco smoke major inhalation source (1. 5 -2 ug/cig) • Water (naturally at <10 ng/l) EPA max 0. 01 mg/l, (goal 5 ug/l) – In salt waters as Cd. Cl 2, in fresh waters as Cd. CO 3 • Soil – Deposition from air – Municipal sewage on agricultural soil – Phosphate fertilizers • Food - 5 -10% retention (10 -50 ng/d to 200 -1000 ug/d) – Main source of human exposure (plants bioaccumulate Cd) – Leafy vegetables, grains, cereals – Some seafoods

Cd Health effects • Known human carcinogen (lung cancer) (air 1 ug/m 3) • Accumulation in liver, kidney (t 1/2=10 -20 y), and skeleton over lifetime - Very low excretion (0. 005%/day) • Nephrotoxicity due to Ca++ ion uptake inhibition (free intracellular Cd ions; Metallothionein) • Mechanisms: Enzyme inhibition, metal co-factor displacement, oxidative damage (lipid peroxidation) • Antagonist of nutritional metal intake (role of deficiencies in toxicity, protein in diet, vit. C) • Newborns and children most sensitive

Mercury - Hg • Unique properties and rare on earth crust, but also ubiquitous • Multiple uses (thermometers, UV light lamps, catalyst, batteries, electrical apparatus) • US sources: chloralkali industry and coal fired power plants (40%); also pulp and paper industry, incineration, smelters, gold mining (Amazon) • Also natural sources (volcano eruptions) • Elemental Hg is oxidized to Hg++ and biotransformed to organic forms (mostly methyl) • Bioaccumulates in fat tissue - fish intake
FDA guideline for fish 0. 5 ug/g Critical daily dose 300 mg Hg Health effects • Brain is target organ: Neurotoxicity, psychomotor effects, brain damage (fetus) • Poisonings – Minamata Bay acute toxicity (Japan) (11 mg/g) – Iraq 1971 -72, bread - Me. Hg as fungicide • Women of childbearing age and children are subpopulations of highest concern • Enzyme inhibition (-SH binding) • Na+ and K+ membrane permeability • Nephrotoxicity • Se protective?
OSHA air level 7 ug/m 3 (occupational) No drinking water safety level Nickel - Ni • Occupational toxicity - inhalation - Ni(CO)4 • Water contamination through leakage • Carcinogenic forms - Ni, Ni 2 S 3, Ni. Ox – DNA and protein crosslinking – Chromosomal aberrations – Oxidative processes • • Competition with essential metals Skin contact exposure Crosses the blood-placenta barrier Mg protective?

EPA safe level 50 ug/l (drinking water) Arsenic -As • Oxides, As. O 3, H 3 As. O 3 • Uses: Insecticides, rodenticides, herbicides, fungicides, preservatives, pigments, vet med. • Sources: Natural processes, fossil fuel combustion (fly ash particulates), smelting (As. H 3) • Microorganismal oxidation, methylation (organic forms) • Groundwater and surface water contamination – 350, 000 US residents above safe level – Mostly in Asia (India, China), South America (Chile) • Urban air (0. 02 ug/m 3), soil (0. 2 -40 ug/g) • Food - fish

Arsenic Health Effects • Inhalation, ingestion, skin contact – Liver, kidneys, spleen, intestine (lung), skin, hair, nails • Toxicity higher for water soluble forms (As 3+), metabolic transformation (methylation detox) • CNS effects (motor activity) • Carcinogen (bladder, kidney, skin, liver, blood, lung and colon (inorganic forms) (0. 35 -1. 14 mg/l) • Capillary injury - “Blackfoot” (gangrene) • Teratogen • Reacts with -SH: enzyme inhibitor (antidote BAL) • Uncouples oxidative phosphorylation • Oxidative processes (SOD, CAT, GPX, GST inhibition)
- Slides: 17