METALS Composition and Microstructure Ferrous Metals and Alloys
































- Slides: 57

METALS Composition and Microstructure Ferrous Metals and Alloys Non-Ferrous Metals and Alloys Specifications and Proof Testing Corrosion

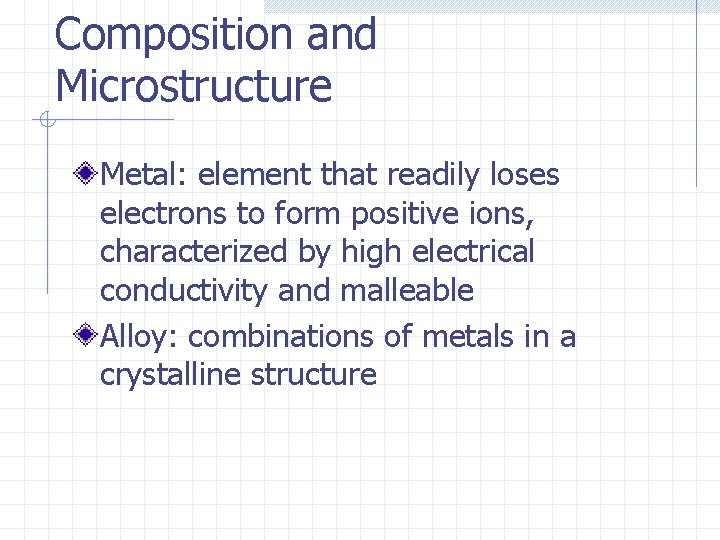
Composition and Microstructure Metal: element that readily loses electrons to form positive ions, characterized by high electrical conductivity and malleable Alloy: combinations of metals in a crystalline structure

Structure of Metals Microstructural properties determine all of the material properties of metals and alloys. Different from Covalent and Ionic Bonds

Alloying Structure 3 -D lattice in metalic bonds provides n n opportunity for other element to occupy some of the positions. or for small element to enter the lattice

Interstitial Alloy Between atomic lattice location < 60% of the size of the lattice atoms only a small % can fit interstitially For Transition metals only a few fit H, B, C, N

Substitutional Alloy Replacing elements in the lattice + 15% radius of lattice atoms large percentage is possible [ Alloys may contain both interstitial and substitutional elements

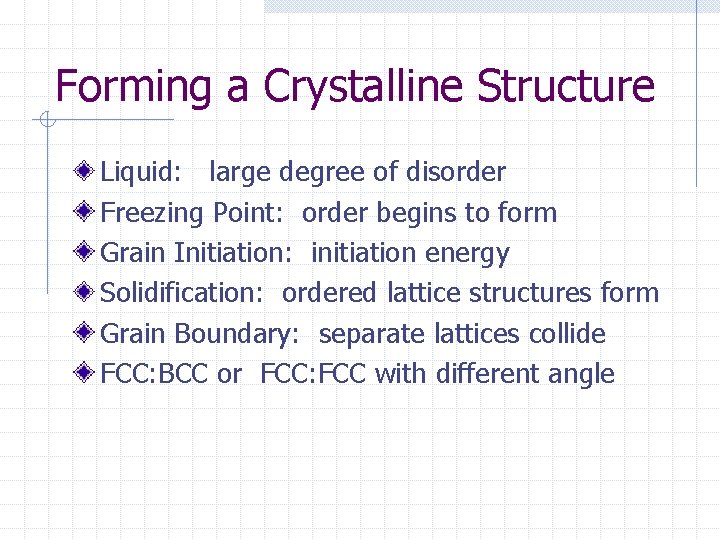
Forming a Crystalline Structure Liquid: large degree of disorder Freezing Point: order begins to form Grain Initiation: initiation energy Solidification: ordered lattice structures form Grain Boundary: separate lattices collide FCC: BCC or FCC: FCC with different angle

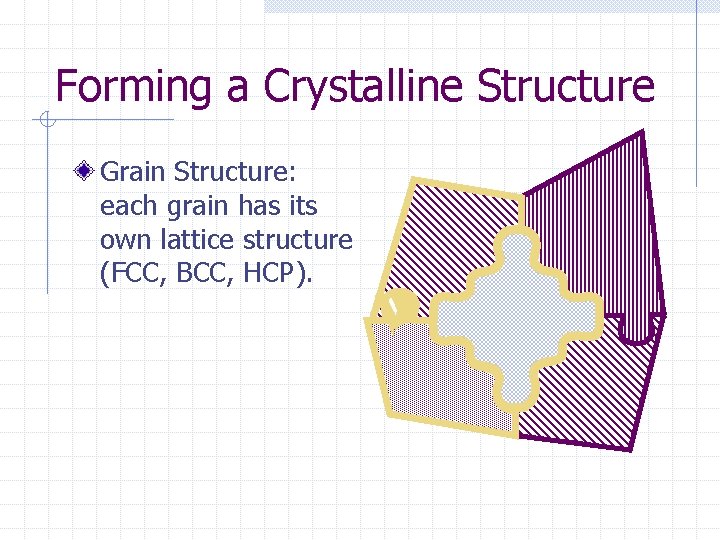
Forming a Crystalline Structure Grain Structure: each grain has its own lattice structure (FCC, BCC, HCP).

Introduction to Steel Production Commercial Forms Applications Microstructure Strengthening Mechanisms Corrosion

Metal Processing Crushing and Calcining, or Separation Extraction n Smelting w Ore is melted and separated in solution n Electrolytic processing w electric furnace or process is used to separate metal n Leaching (liquid processing) w metal is recovered from leachate

Ferrous Metals principle element is iron, cast iron, steel, wrought iron. Metals come from ore, "minerals" ore consists of metal and gangue (valueless extra) Mining n n open pit underground

Refining the Metal n n oxidizing impurities distillation chemical agents electrolysis

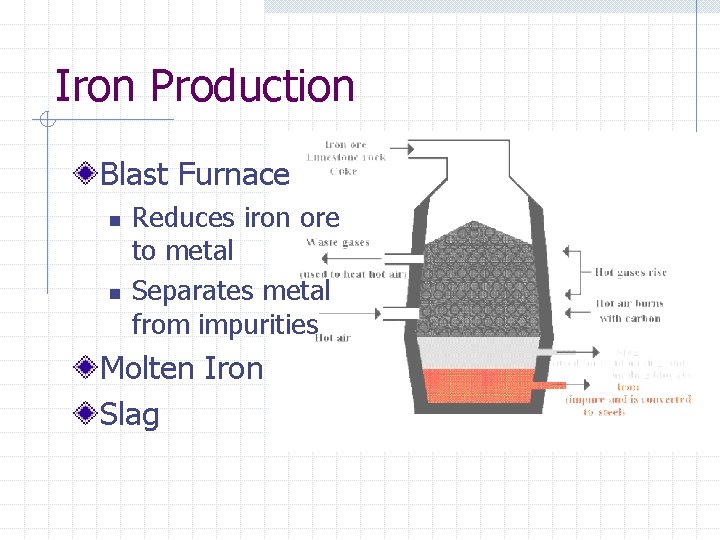
Iron Production Blast Furnace n n Reduces iron ore to metal Separates metal from impurities Molten Iron Slag

Processing of Virgin Steel 1) first step in reducing iron ore, 2) separates impurities 3) absorbs carbon (leaves 2. 5 - 4. 5 % carbon) End product is cast in bars, "pigs".

Ferrous Metals Pig Iron n Iron ore is combined with coke, and limestone (fluxing agent). Blasts of hot air are forced through the material to ignite the coke and melt the iron ore. The impurities in the iron are absorbed by the limestone and forms blast furnace slag.

Forms of Ferrous Alloys Cast Iron n cast iron is pig iron is any other shape. Remelted and cast into desired shape. Malleable Cast Iron n annealed (heating then slow cooling to encourage refined grains and soften mechanical properties, removes internal stresses, removes gases) cast iron that has been made more ductile and formable.

Forms of Ferrous Alloys Wrought Iron n a form of iron that contains slag, and virtually no carbon. making it workable when it is hot but hardens very rapidly when cooled rapidly. Ingot Iron n low carbon steel or iron cast from a molten state.

Forms of Ferrous Alloys Steel n n Iron - Carbon alloy which is cast from a molten mass in a form which is malleable. Carbon steel is steel with less than 1. 5% carbon. Alloy steel is steel which has properties controlled by elements other than carbon. Steel has the best structural properties of these materials

Carbon Steels Carbon steels have between. 008 and 1. 7 percent C (most are between 0. 1 and 0. 8%) Carbon may be substitutional or interstitial depending upon the amount present Alloys with greater than 1. 7 percent carbon become very brittle and hard, i. e. cast iron properties.

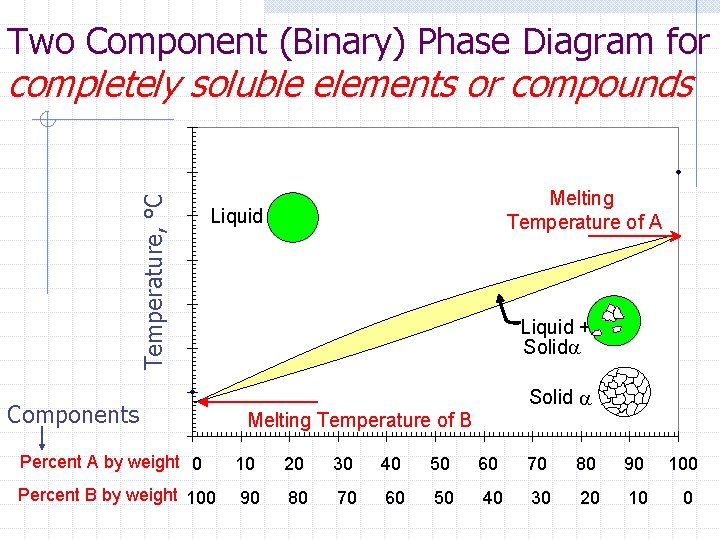
Phase Diagrams relate the n n composition & temperature to the crystalline structure (“phase”) Inverse Lever Law n determines the percentage of each crystalline phase

Two Component (Binary) Phase Diagram for Temperature, °C completely soluble elements or compounds Melting Temperature of A Liquid Components Liquid + Solida Solid a Melting Temperature of B Percent A by weight 0 10 20 30 40 50 60 70 80 90 100 Percent B by weight 100 90 80 70 60 50 40 30 20 10 0

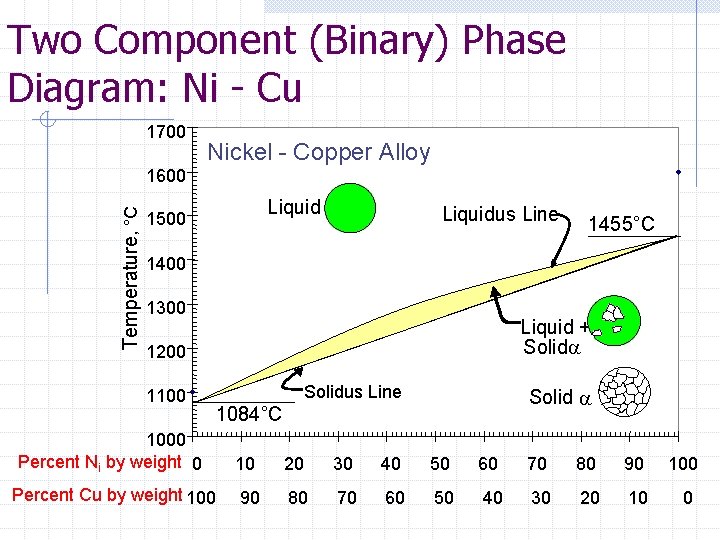
Two Component (Binary) Phase Diagram: Ni - Cu 1700 Nickel - Copper Alloy Temperature, °C 1600 Liquid 1500 Liquidus Line 1455°C 1400 1300 Liquid + Solida 1200 1100 Solidus Line Solid a 1084°C 1000 Percent Ni by weight 0 10 20 30 40 50 60 70 80 90 100 Percent Cu by weight 100 90 80 70 60 50 40 30 20 10 0

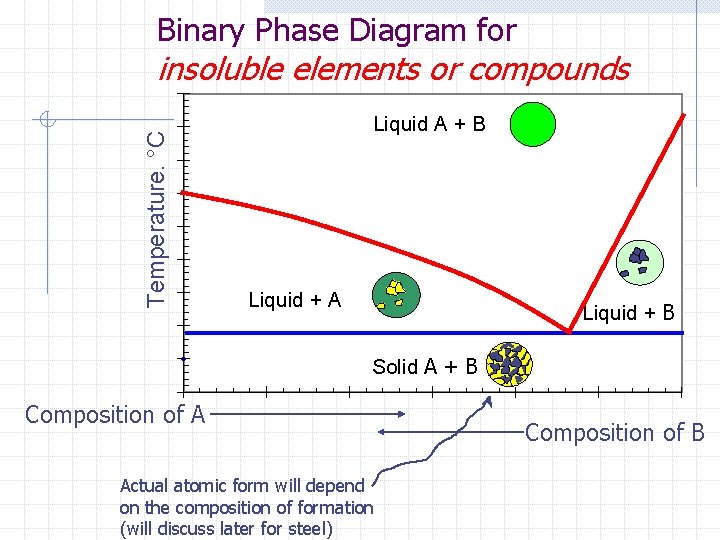
Binary Phase Diagram for Temperature. °C insoluble elements or compounds Liquid A + B Liquid + A Liquid + B Solid A + B Composition of A Actual atomic form will depend on the composition of formation (will discuss later for steel) Composition of B

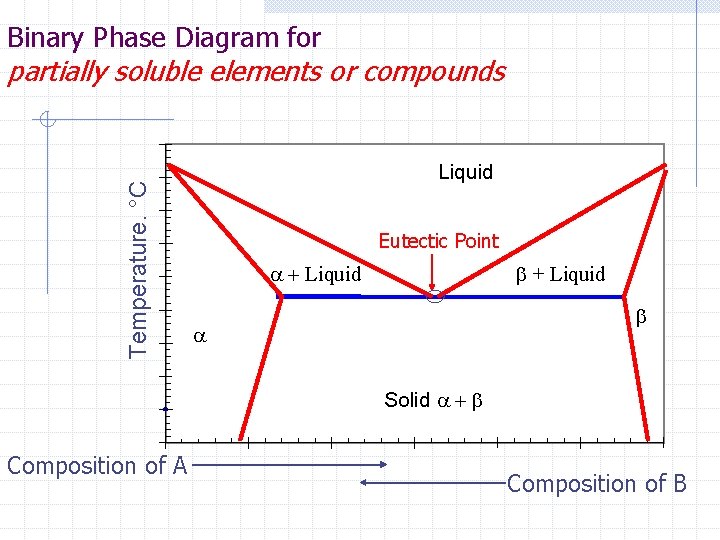
Definitions Eutectic Reaction – Eutectic Point – Eutectic Solid –

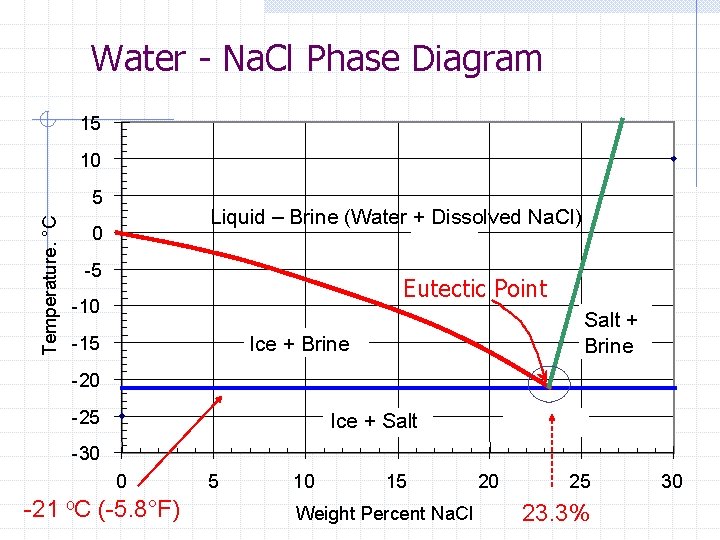
Water - Na. Cl Phase Diagram 15 10 Temperature. °C 5 Liquid – Brine (Water + Dissolved Na. Cl) 0 -5 Eutectic Point -10 Salt + Brine Ice + Brine -15 -20 -25 Ice + Salt -30 0 -21 o. C (-5. 8°F) 5 10 15 Weight Percent Na. Cl 20 25 23. 3% 30

Binary Phase Diagram for Temperature. °C partially soluble elements or compounds Liquid Eutectic Point a + Liquid b a Solid a + b Composition of A Composition of B

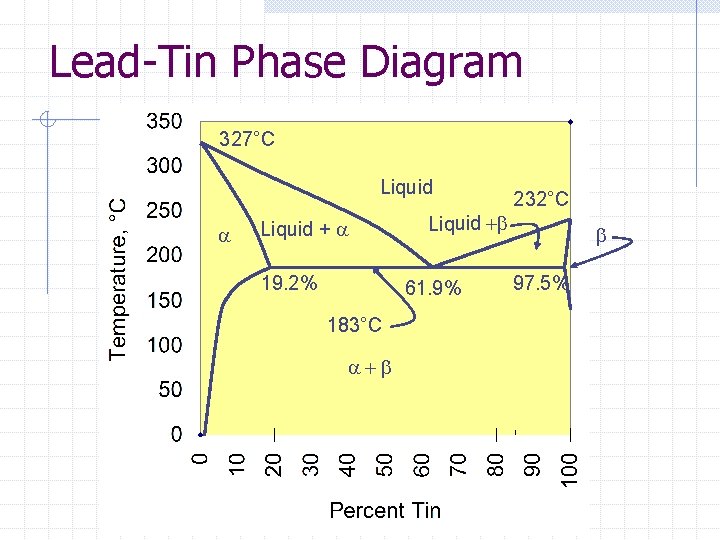
Lead-Tin Phase Diagram 327°C Liquid a Liquid + a 19. 2% Liquid +b 61. 9% 183°C a+b 232°C b 97. 5%

Definitions Eutectoid Reaction – Eutectoid Point – Eutectoid –

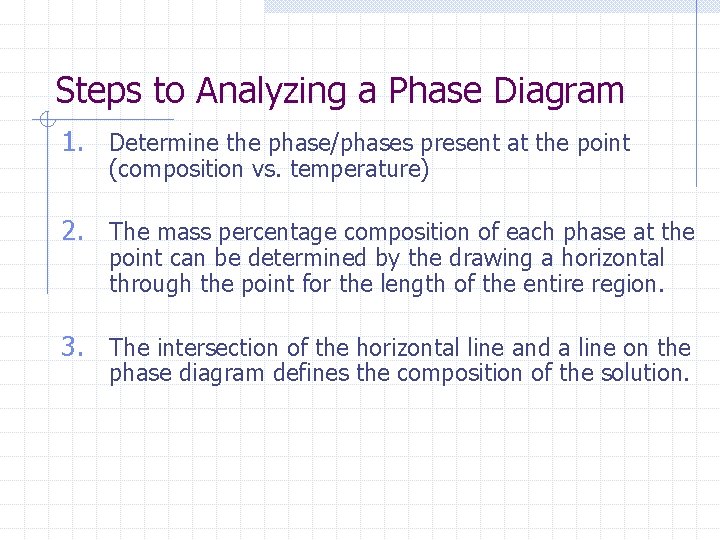
Steps to Analyzing a Phase Diagram 1. Determine the phase/phases present at the point (composition vs. temperature) 2. The mass percentage composition of each phase at the point can be determined by the drawing a horizontal through the point for the length of the entire region. 3. The intersection of the horizontal line and a line on the phase diagram defines the composition of the solution.

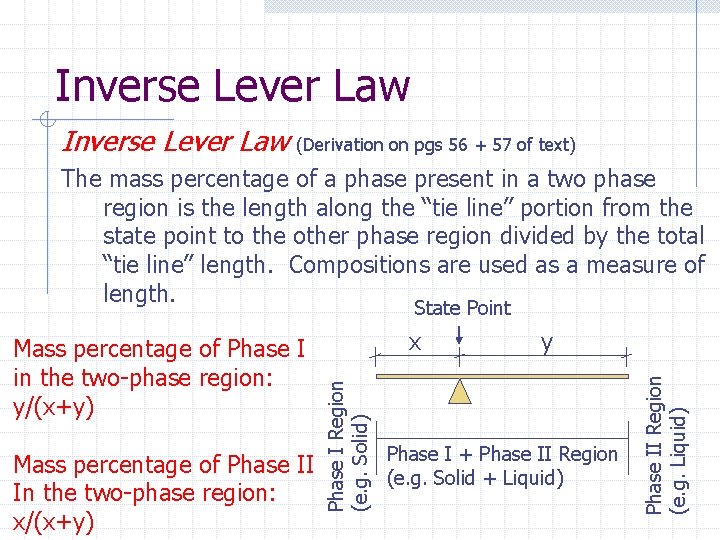
A Point with 2 Phases 4. If the point is located in a region with more 2 phases, the mass percentage of each phase within the region can be determined by the inverse lever law.

Inverse Lever Law (Derivation on pgs 56 + 57 of text) The mass percentage of a phase present in a two phase region is the length along the “tie line” portion from the state point to the other phase region divided by the total “tie line” length. Compositions are used as a measure of length. State Point y Phase I + Phase II Region (e. g. Solid + Liquid) Phase II Region (e. g. Liquid) Mass percentage of Phase II In the two-phase region: x/(x+y) x Phase I Region (e. g. Solid) Mass percentage of Phase I in the two-phase region: y/(x+y)

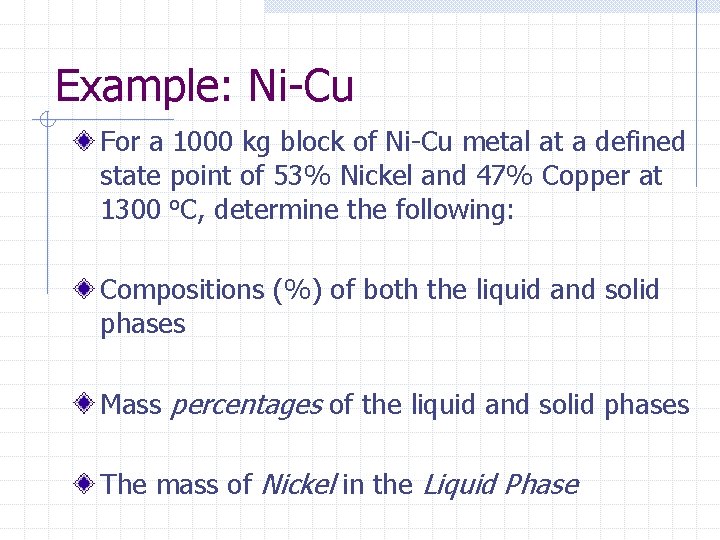
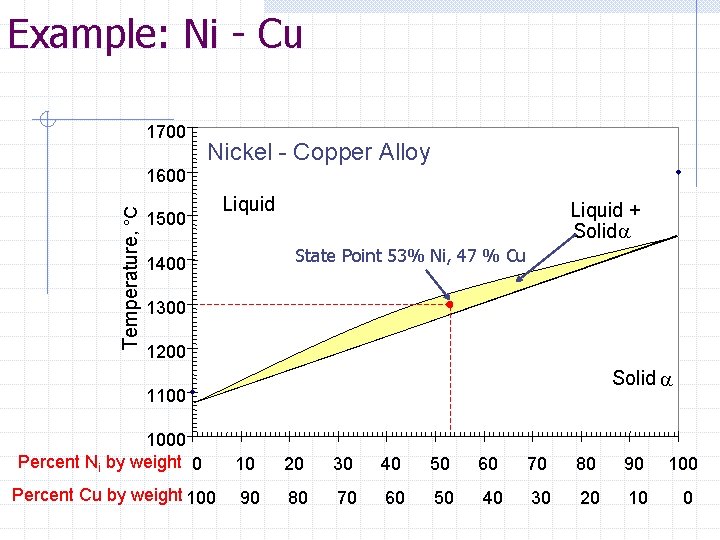
Example: Ni-Cu For a 1000 kg block of Ni-Cu metal at a defined state point of 53% Nickel and 47% Copper at 1300 o. C, determine the following: Compositions (%) of both the liquid and solid phases Mass percentages of the liquid and solid phases The mass of Nickel in the Liquid Phase

Example: Ni - Cu 1700 Nickel - Copper Alloy Temperature, °C 1600 1500 Liquid + Solida State Point 53% Ni, 47 % Cu 1400 1300 1200 Solid a 1100 1000 Percent Ni by weight 0 10 20 30 40 50 60 70 80 90 100 Percent Cu by weight 100 90 80 70 60 50 40 30 20 10 0

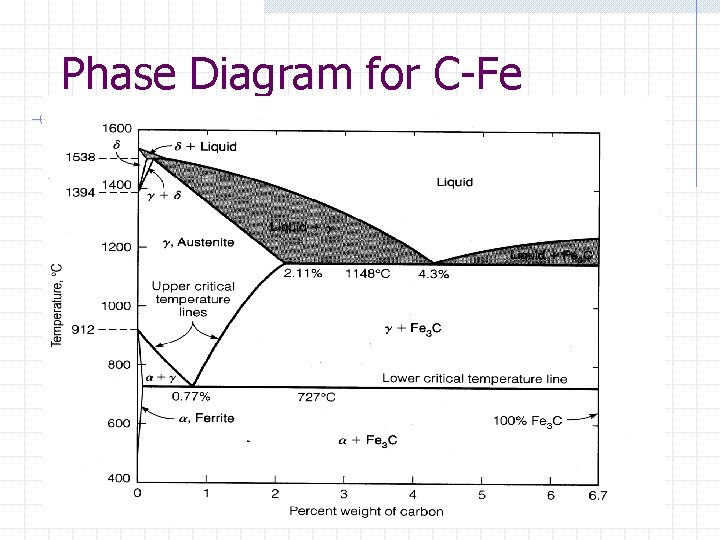
Phase diagram for Fe-C Cementite: n n n above 4. 35 to 6. 67 very hard and brittle alloy forms 6. 67% Carbon 93. 33% Iron "iron carbide" Ferrite: n iron which contains very little carbon. this is soft ductile material

Phase diagram for Fe-C Pearlite: n n combination of ferrite and cementite structures intermediate property structure Austinite: n solid state gamma phase iron-carbon combination.

Phase Diagram for C-Fe

Microstructure Phases of Steel n n Ferrite (BCC) Austenite (FCC) Cementite (Orthorhombic) Delta Iron (BCC) Grain Size


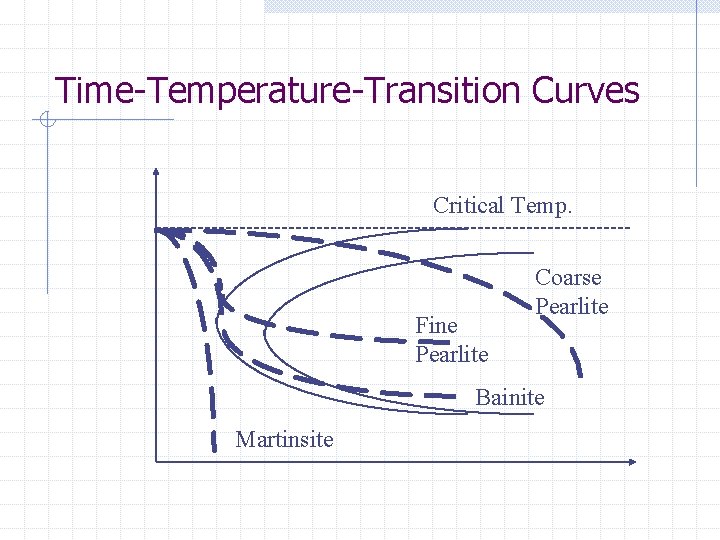
Time-Temperature-Transition Curves Critical Temp. Fine Pearlite Coarse Pearlite Bainite Martinsite

Heat Treatments Annealing n n n heated above critical temperature and cooled slowly softens structure Quenching n n n heated above critical temperature and cooled rapidly in water or oil improves hardness and strength

Heat Treatments Tempering n n n heated below critical temperature, held and quenched improves ductility and toughness while retaining hardness



Mild Steel Grades A 992 “Low Alloy” Carbon Steel n n n <0. 23% Carbon Common Structural Sections Replaced A 36 steel A 572 “High-Strength Low-Alloy Columbium-Vanadium Steel” n n Grades 42, 50, 65 Structural sections and bolts. . .

Mild Steel Grades A 615 Billet Reinforcing Steel n n low alloy, high ductility steel reinforcing bars A 588 Weathering Steel n n should not be used in Cl water environments Free from moisture 40% of the time; avoid extreme humid environments

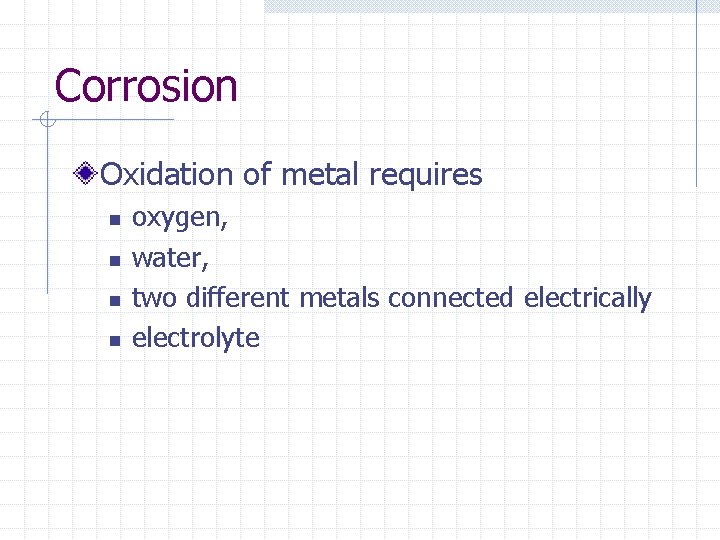
Corrosion Oxidation of metal requires n n oxygen, water, two different metals connected electrically electrolyte

Corrosion Major problem with steel Control Methods n n Protective Coatings Galvanic Protection Cathodic Protection Corrosion-resistant Steels

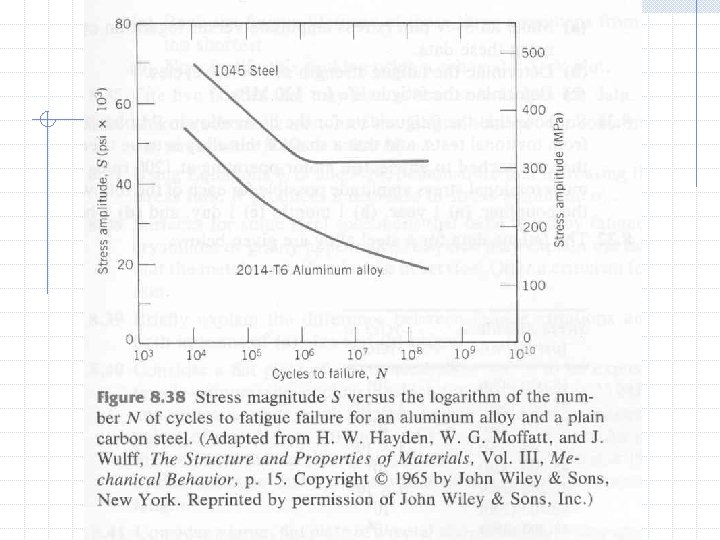
S-N Curve

Strengthening Mechanisms Alloying Heat Treating Cold Working

Alloying Forming Solid Solution with Iron n Carbon, Chromium, Manganese, Nickel, Copper, and Silicon Formation of Carbide n Titanium, Vanadium, and Molybdenum Formation of an Undissolved, second phase n Lead, Sulfur, and Phosphorus

Heat Treatments Full Annealing Process Annealing Normalizing Quenching

Cold Working Plastic deformation Done below recrystallization temperature

Other Properties of Steel Impact n resistance to dynamic loadings (toughness) Creep n time dependent deformation due to sustained loads Ductility n n mild steels may yield at = 0. 002 and fracture at > 0. 200

Forms of Steel Structural Shapes n n Wide flange sections, Channels, Tubing, Plate Reinforcing Steel Cold Rolled forms, pans, sheet Pipe

Structural Grades ASTM n n n A 36 & A 572 (being phased out) A 992 Structural Shapes A 325 Bolts AISI - SAE n 10 XX w XX defines Carbon content n 13 XX w 13 defines a manganese alloy steel

Applications Structural Members Bolts, Connectors Reinforcement Tools Machines

Steel Grades