Metals and their Extraction WJEC Chemistry Unit 2


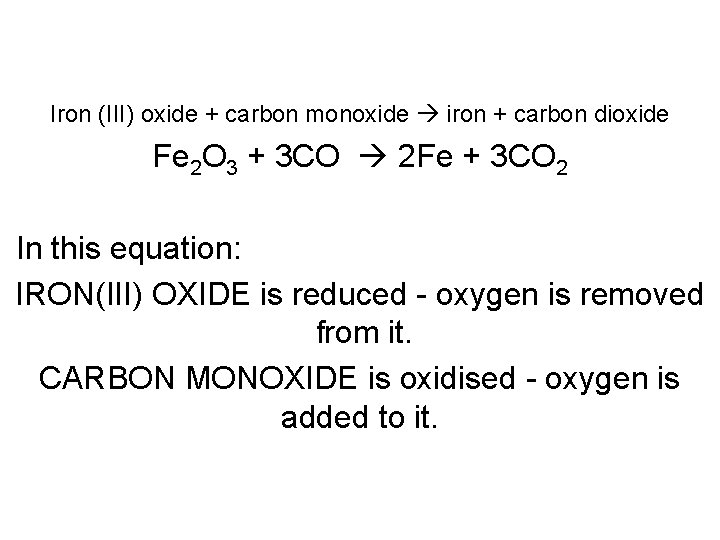

- Slides: 18

Metals and their Extraction WJEC Chemistry Unit 2 - 2. 3 WJEC Science (Double Award) Unit 5 - 5. 3

Metals and Ores The least reactive metals occur in an uncombined form within the Earth’s crust. e. g. gold The other metals exist as ores in combination with other elements such as oxygen and sulphur. Haematite Pyrite (a mineral of iron - oxide) (a mineral of iron sulphide) Iron pan Most metals are produced from their ores by a process called reduction. This is where oxygen is removed from a substance.

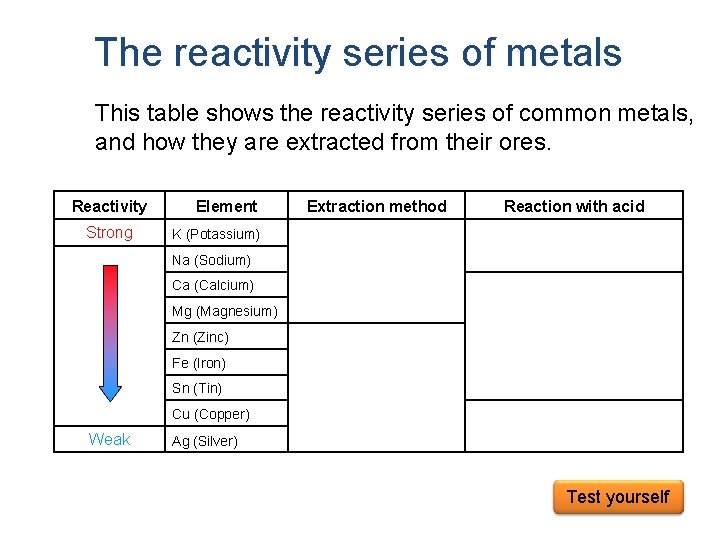
The reactivity series of metals This table shows the reactivity series of common metals, and how they are extracted from their ores. Reactivity Strong Element Extraction method K (Potassium) Na (Sodium) Ca (Calcium) Reaction with acid Too dangerous Electrolysis Mg (Magnesium) Releases hydrogen with dilute hydrochloric acid. Zn (Zinc) Fe (Iron) Sn (Tin) Cu (Copper) Weak Ag (Silver) Chemical reduction Does not release hydrogen with dilute hydrochloric acid. Test yourself

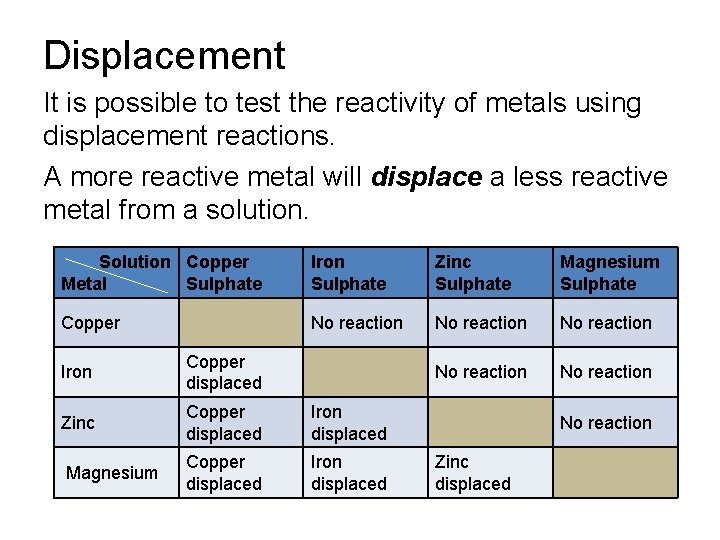
Displacement It is possible to test the reactivity of metals using displacement reactions. A more reactive metal will displace a less reactive metal from a solution. Solution Copper Metal Sulphate Iron Sulphate Zinc Sulphate Magnesium Sulphate Copper No reaction No reaction Iron Copper displaced Zinc Copper displaced Iron displaced Magnesium Copper displaced Iron displaced No reaction Zinc displaced

Competition reactions A more reactive metal will remove oxygen from the oxide of a less reactive metal when a mixture of the two is heated. This is a competition reaction. When a mixture of powdered aluminium and iron(III) oxide is ignited by a high temperature fuse, molten iron is formed as the aluminium is more reactive metal and so removes the oxygen from iron (III) oxide. This reaction is called thermit reaction and is used to weld sections of railway track. A mixture of aluminium and iron(III) oxide reacting together railway track crucible railway track Reduction – iron (III) oxide iron Oxidation – aluminium oxide

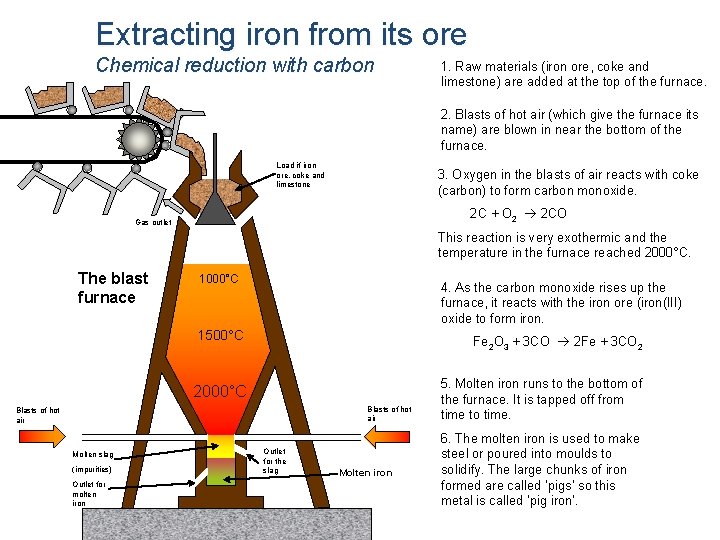
Extracting iron from its ore Chemical reduction with carbon 1. Raw materials (iron ore, coke and limestone) are added at the top of the furnace. 2. Blasts of hot air (which give the furnace its name) are blown in near the bottom of the furnace. Load if iron ore, coke and limestone 3. Oxygen in the blasts of air reacts with coke (carbon) to form carbon monoxide. 2 C + O 2 2 CO Gas outlet This reaction is very exothermic and the temperature in the furnace reached 2000°C. The blast furnace 1000°C 4. As the carbon monoxide rises up the furnace, it reacts with the iron ore (iron(III) oxide to form iron. 1500°C Fe 2 O 3 + 3 CO 2 Fe + 3 CO 2 2000°C Blasts of hot air Molten slag (impurities) Outlet for molten iron Outlet for the slag Molten iron 5. Molten iron runs to the bottom of the furnace. It is tapped off from time to time. 6. The molten iron is used to make steel or poured into moulds to solidify. The large chunks of iron formed are called ‘pigs’ so this metal is called ‘pig iron’.
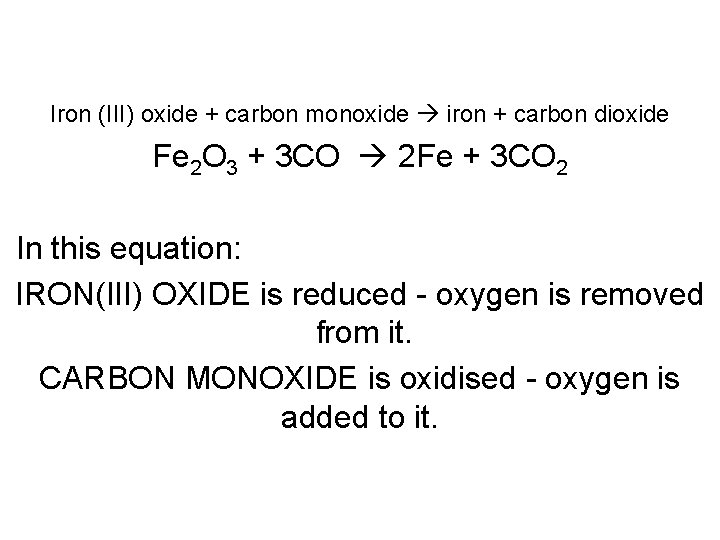
Iron (III) oxide + carbon monoxide iron + carbon dioxide Fe 2 O 3 + 3 CO 2 Fe + 3 CO 2 In this equation: IRON(III) OXIDE is reduced - oxygen is removed from it. CARBON MONOXIDE is oxidised - oxygen is added to it.

Steel – properties and uses Iron from the blast furnace is very brittle because it contains up to 4. 5% carbon. Most of this iron is converted to steel, which is far more useful, by removing most of the carbon. Mild steel contains approximately 0. 5% carbon Hard steel contains up to 1. 5% carbon. Car bodies machines It is possible to treat steel. Heat treatment Domestic appliances Creating alloys with other metals Stainless steel (chromium and nickel) Very hard steel (tungsten) Tough steel (manganese) Tinplate

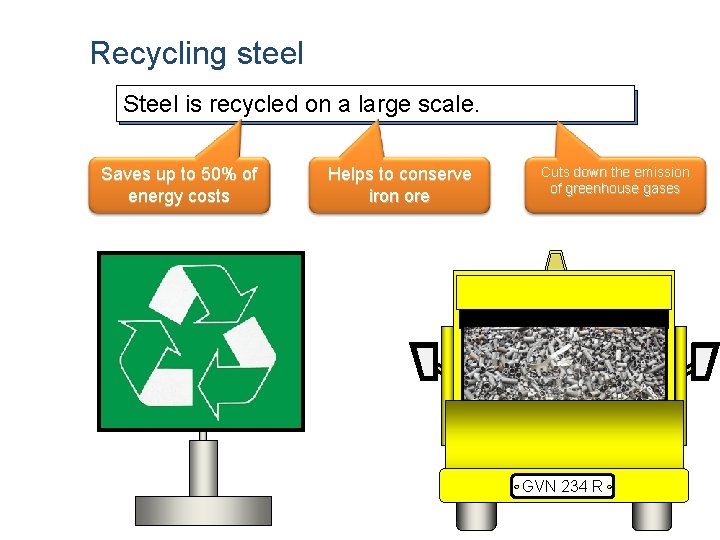
Recycling steel Steel is recycled on a large scale. Saves up to 50% of energy costs Helps to conserve iron ore Cuts down the emission of greenhouse gases GVN 234 R

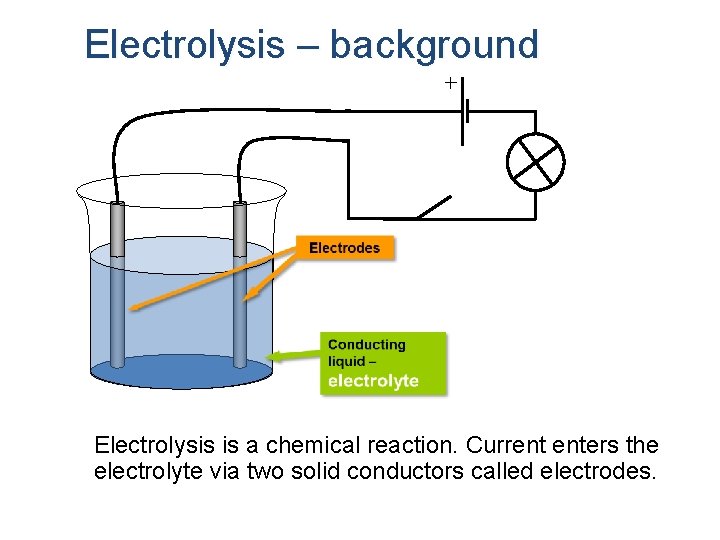
Electrolysis – background Electrolysis is a chemical reaction. Current enters the electrolyte via two solid conductors called electrodes.

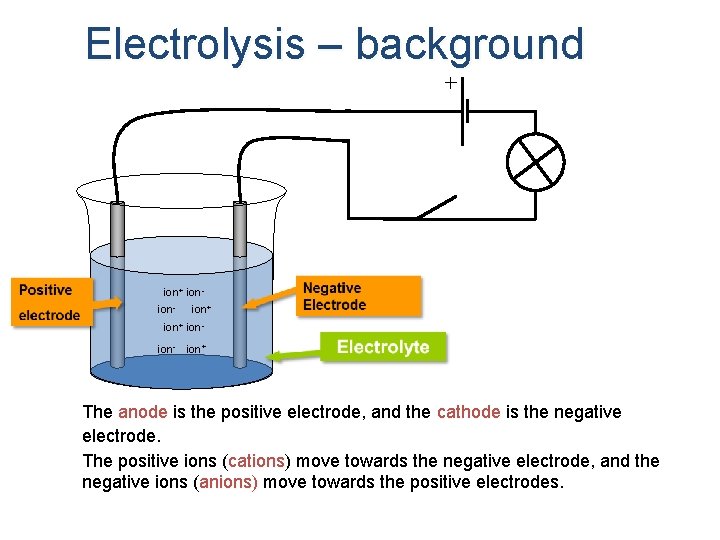
Electrolysis – background ion+ ionion- ion+ The anode is the positive electrode, and the cathode is the negative electrode. The positive ions (cations) ions move towards the negative electrode, and the negative ions (anions) move towards the positive electrodes.

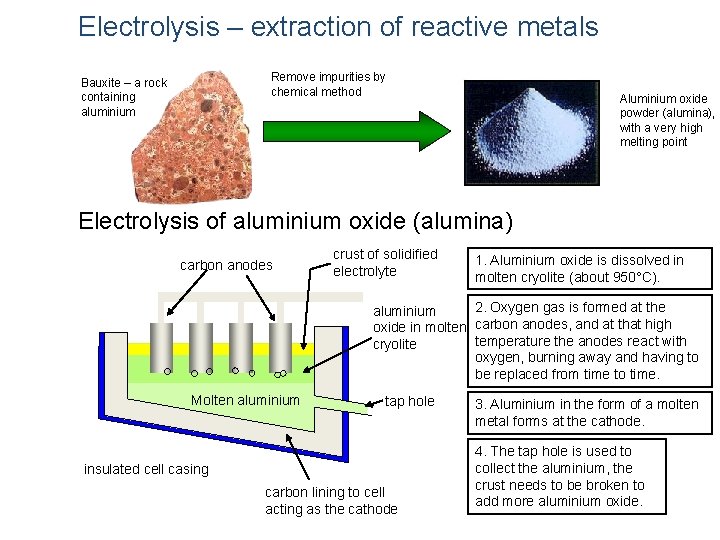
Electrolysis – extraction of reactive metals Remove impurities by chemical method Bauxite – a rock containing aluminium Aluminium oxide powder (alumina), with a very high melting point Electrolysis of aluminium oxide (alumina) carbon anodes crust of solidified electrolyte 1. Aluminium oxide is dissolved in molten cryolite (about 950°C). 2. Oxygen gas is formed at the aluminium oxide in molten carbon anodes, and at that high temperature the anodes react with cryolite oxygen, burning away and having to be replaced from time to time. Molten aluminium tap hole insulated cell casing carbon lining to cell acting as the cathode 3. Aluminium in the form of a molten metal forms at the cathode. 4. The tap hole is used to collect the aluminium, the crust needs to be broken to add more aluminium oxide.

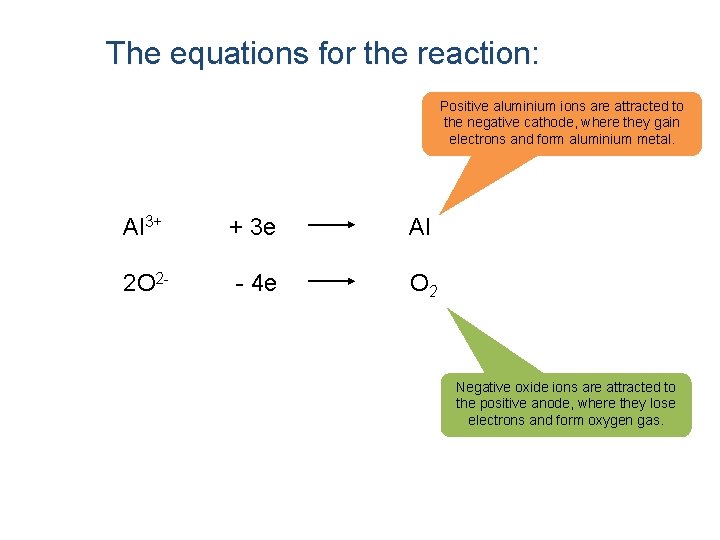
The equations for the reaction: Positive aluminium ions are attracted to the negative cathode, where they gain electrons and form aluminium metal. Al 3+ + 3 e Al 2 O 2 - - 4 e O 2 Negative oxide ions are attracted to the positive anode, where they lose electrons and form oxygen gas.

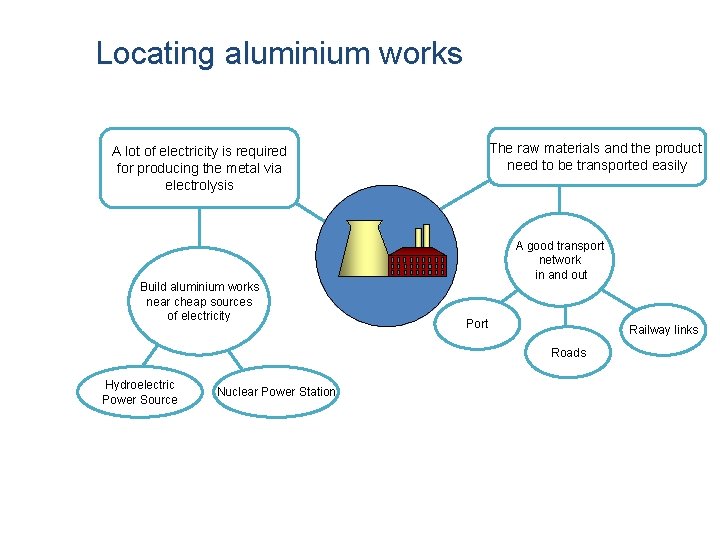
Locating aluminium works The raw materials and the product need to be transported easily A lot of electricity is required for producing the metal via electrolysis Build aluminium works near cheap sources of electricity A good transport network in and out Port Railway links Roads Hydroelectric Power Source Nuclear Power Station

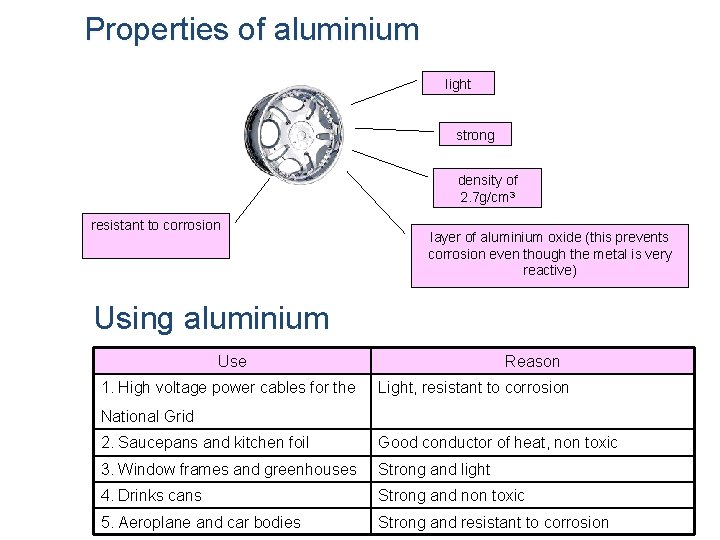
Properties of aluminium light strong density of 2. 7 g/cm 3 resistant to corrosion layer of aluminium oxide (this prevents corrosion even though the metal is very reactive) Using aluminium Use 1. High voltage power cables for the Reason Light, resistant to corrosion National Grid 2. Saucepans and kitchen foil Good conductor of heat, non toxic 3. Window frames and greenhouses Strong and light 4. Drinks cans Strong and non toxic 5. Aeroplane and car bodies Strong and resistant to corrosion

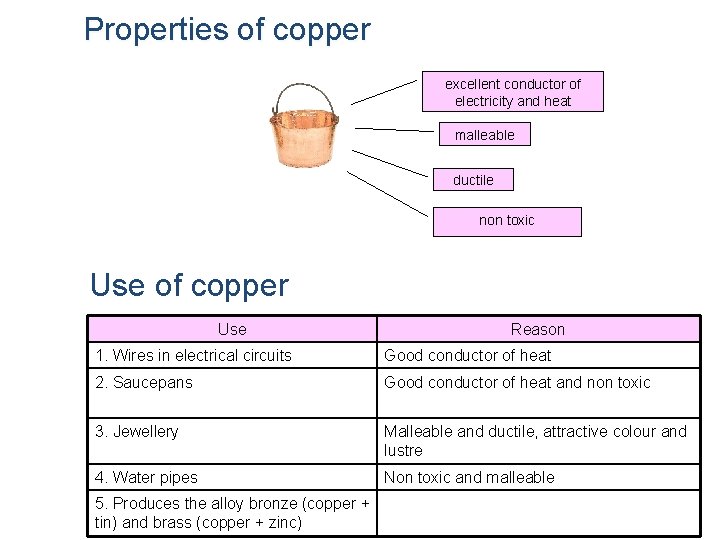
Properties of copper excellent conductor of electricity and heat malleable ductile non toxic Use of copper Use Reason 1. Wires in electrical circuits Good conductor of heat 2. Saucepans Good conductor of heat and non toxic 3. Jewellery Malleable and ductile, attractive colour and lustre 4. Water pipes Non toxic and malleable 5. Produces the alloy bronze (copper + tin) and brass (copper + zinc)

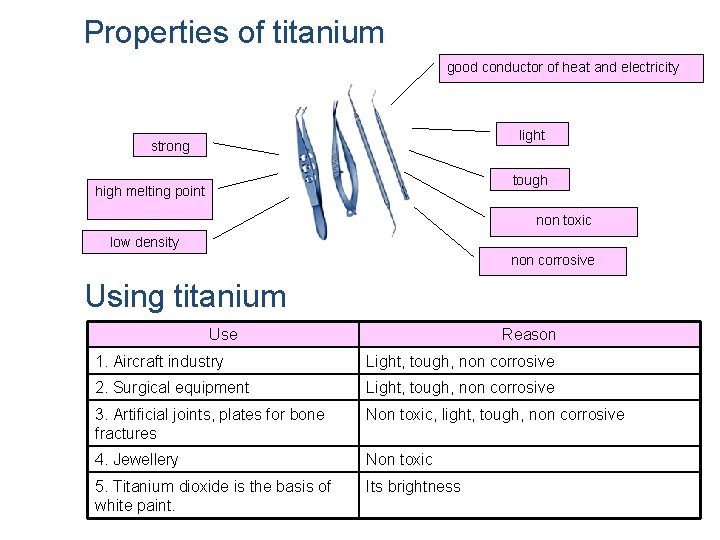
Properties of titanium good conductor of heat and electricity light strong tough high melting point non toxic low density non corrosive Using titanium Use Reason 1. Aircraft industry Light, tough, non corrosive 2. Surgical equipment Light, tough, non corrosive 3. Artificial joints, plates for bone fractures Non toxic, light, tough, non corrosive 4. Jewellery Non toxic 5. Titanium dioxide is the basis of white paint. Its brightness

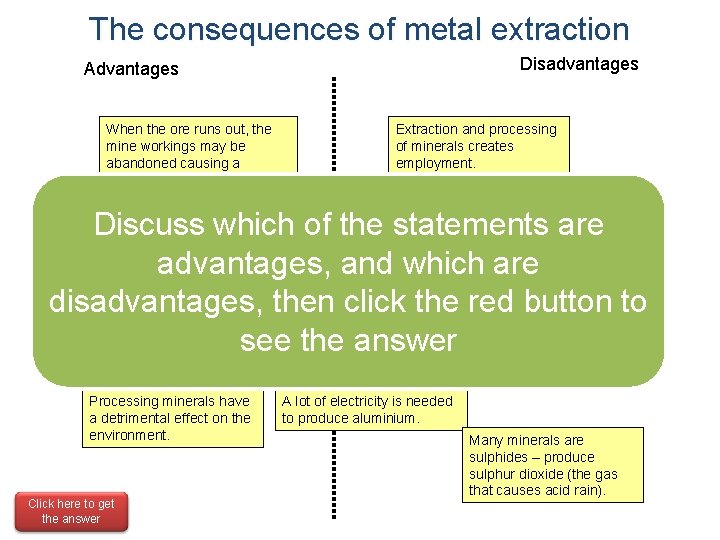
The consequences of metal extraction Disadvantages Advantages When the ore runs out, the mine workings may be abandoned causing a deterioration of the landscape. Extraction and processing of minerals creates employment. Discuss which of the statements are Importing metals is very expensive for the country. advantages, and which are forclick the economy disadvantages, Important then the red button to (Economic boom in the UK Fluctuations in the cost of during Industrial see the answer metals can affect the Revolution). Sometimes less useful and toxic metals are found with the ones that are needed. economy. Processing minerals have a detrimental effect on the environment. Click here to get the answer A lot of electricity is needed to produce aluminium. Many minerals are sulphides – produce sulphur dioxide (the gas that causes acid rain).