Metals and nonmetals The Basics Reactivity Reactions with

Metals and nonmetals The Basics Reactivity Reactions with metals
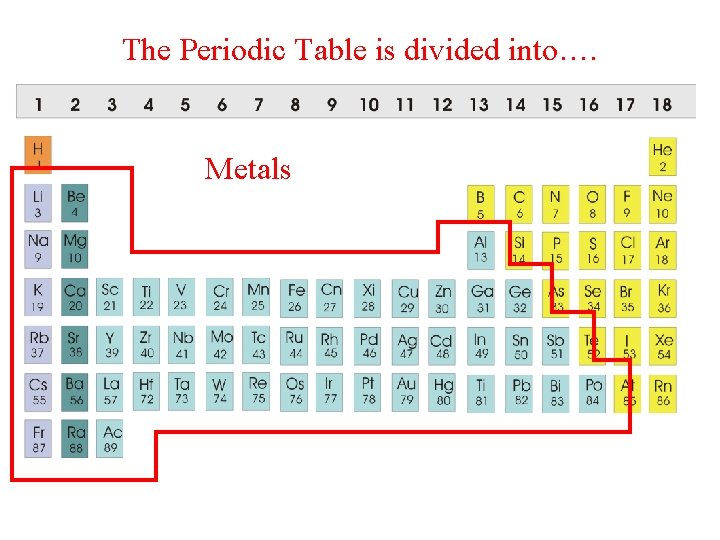
The Periodic Table is divided into…. Metals
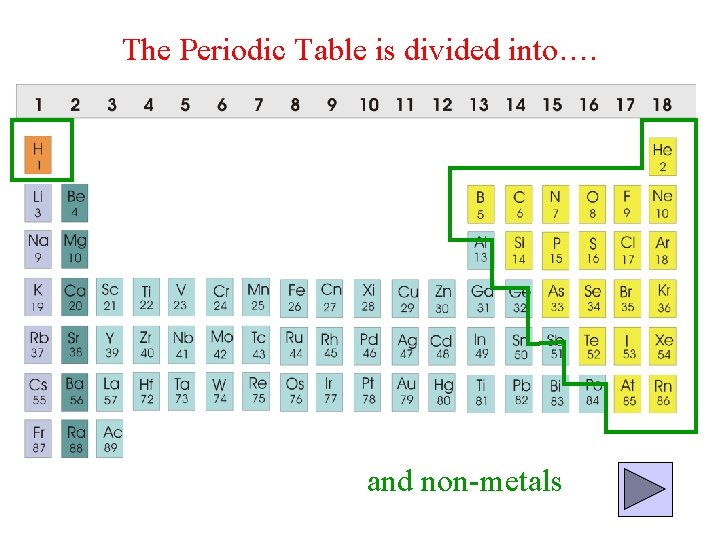
The Periodic Table is divided into…. and non-metals

Metals all have similar physical properties… • They have high melting and boiling points (except mercury) • They conduct electricity and heat. • They have a high density. • They are shiny (lustrous). • They are malleable (can be moulded) • They are ductile (can be stretched)

Non-metals…. • Have low melting and boiling points. • Are brittle. • Do not conduct heat or electricity well. (except carbon)

Different metals are suitable for different jobs. Gold, silver and platinum are very unreactive but very malleable - making them suitable for jewellery

Copper is malleable, ductile and it conducts heat and electricity well. It is also unreactive making it suitable for holding and carrying water.

Aluminium is a reactive metal, but it forms a coating of very stable aluminium oxide. This, together with its lightness and malleability gives it many uses from window frames to insulation foil.

Iron ore is common and iron itself is very strong. It can be moulded into many shapes

As it forms, iron oxide constantly flakes away exposing more of the iron underneath. Remember: Unfortunately iron is quite brittle (for a metal) and also oxidises (rusts) easily. Only iron oxide is called rust. No other metal rusts!

Like aluminium, zinc oxidises to form a stable coating of zinc oxide which protects the iron underneath it. This is called galvanising. To prevent this happening, iron is often covered with a thin layer of zinc.

Lead is very dense, unreactive and malleable - making it suitable for fishing weights and roof sealing. Lead fumes have been linked to brain damage so it is now considered too toxic to be used inside houses (e. g. as in old-fashioned water pipes) Back

Sodium, Calcium and Magnesium These three metals are all far too reactive to be much use in their pure form. They all make important compounds though.

Sodium metal is so reactive it has to be kept in oil to keep it away from the oxygen in the air. . Without the oil, sodium quickly oxides into useless sodium oxide All the same, sodium is essential for both plants and animals. The commonest source of sodium is common table salt – Sodium Chloride.

Calcium is less reactive than sodium, but it still oxidises too rapidly for the pure metal to have any uses. Calcium compounds, however, are very important. Calcium carbonate makes up about 10% of the earth’s surface – limestone, marble and chalk. Calcium compounds are essential for life – such as for making bones and milk.

Magnesium is less reactive then sodium or calcium. It is still too reactive to have any uses in its pure form though. Magnesium is easily obtainable from salts in sea water Amongst other things, magnesium is essential for making chlorophyll.

…. so Group 1 metals are the most reactive Reactivity Metals get more reactive as you move left in the periodic table
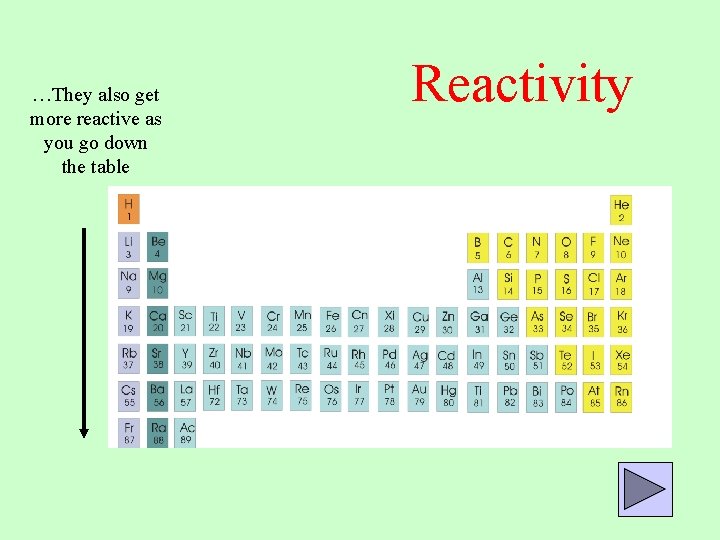
…They also get more reactive as you go down the table Reactivity
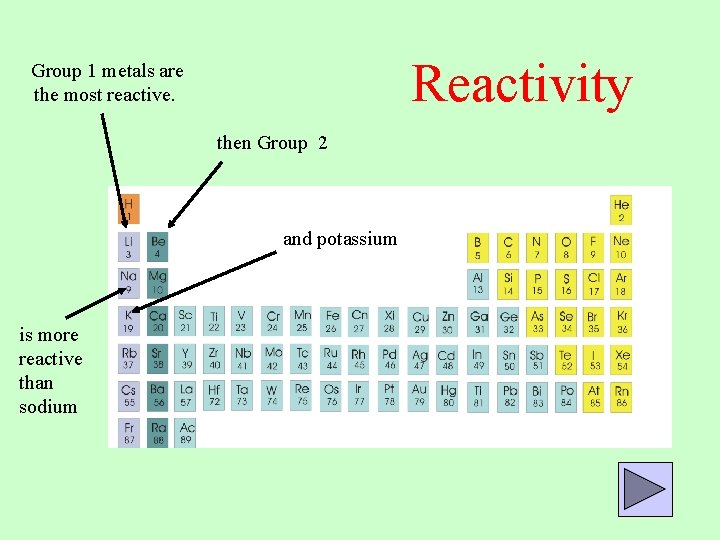
Reactivity Group 1 metals are the most reactive. then Group 2 and potassium is more reactive than sodium
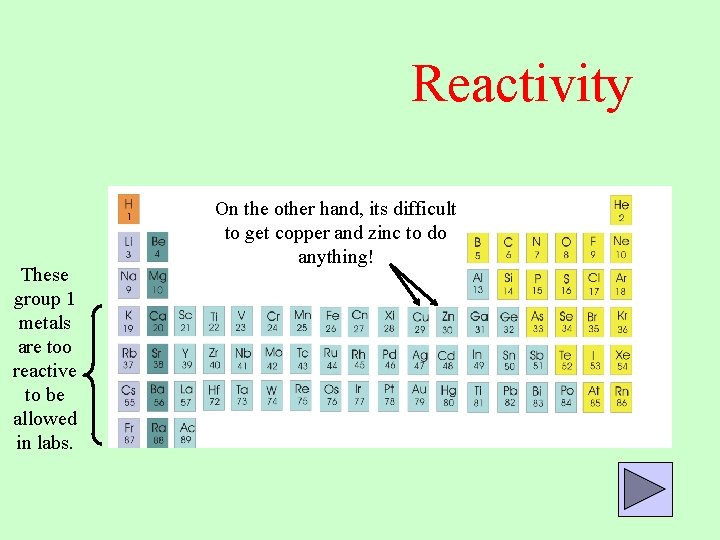
Reactivity These group 1 metals are too reactive to be allowed in labs. On the other hand, its difficult to get copper and zinc to do anything!

Metals all form (They do this by LOSING electrons)

Most non- metals form (They do this by GAINING electrons)
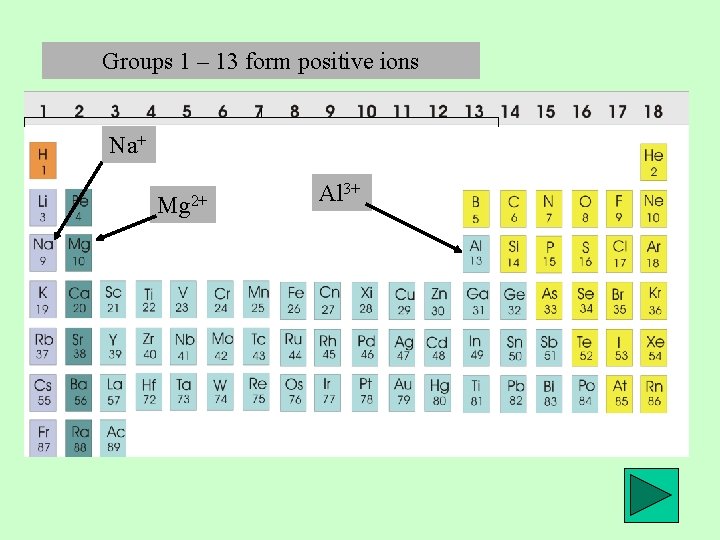
Groups 1 – 13 form positive ions Na+ Mg 2+ Al 3+
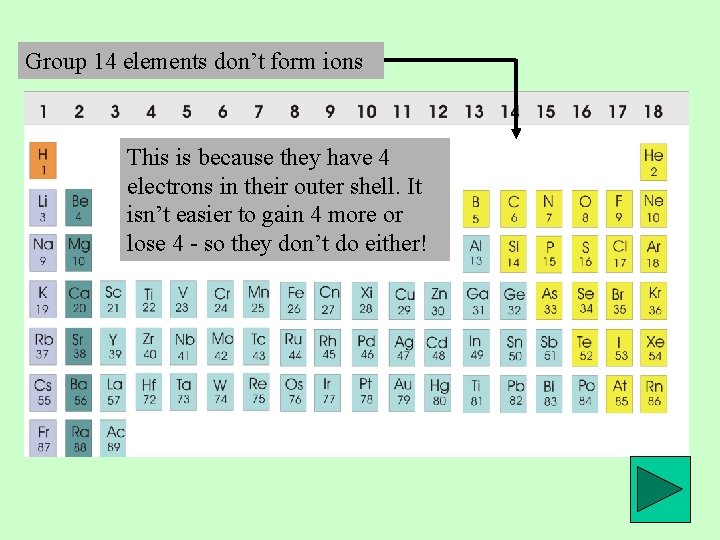
Group 14 elements don’t form ions This is because they have 4 electrons in their outer shell. It isn’t easier to gain 4 more or lose 4 - so they don’t do either!
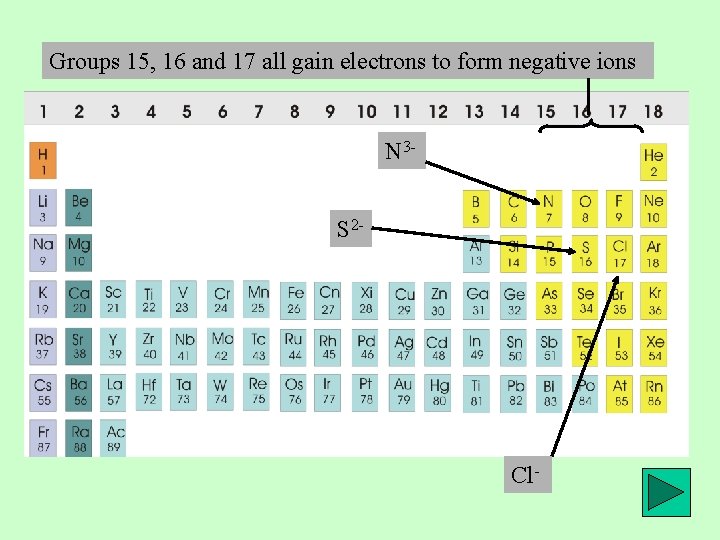
Groups 15, 16 and 17 all gain electrons to form negative ions N 3 S 2 - Cl-
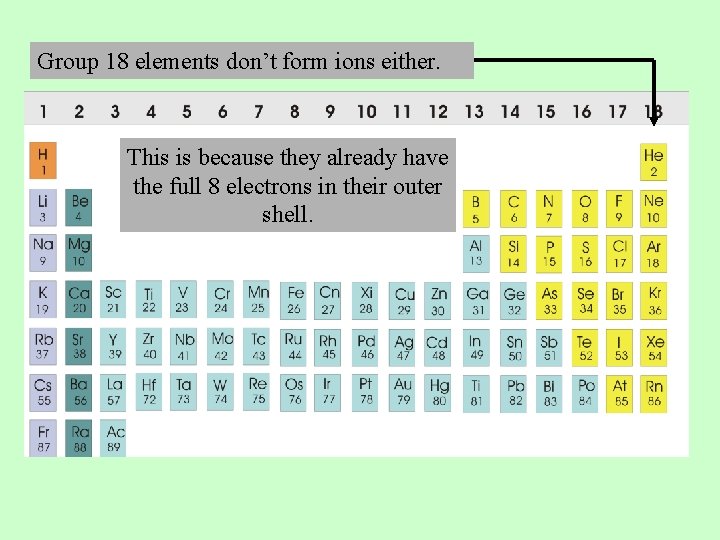
Group 18 elements don’t form ions either. This is because they already have the full 8 electrons in their outer shell.
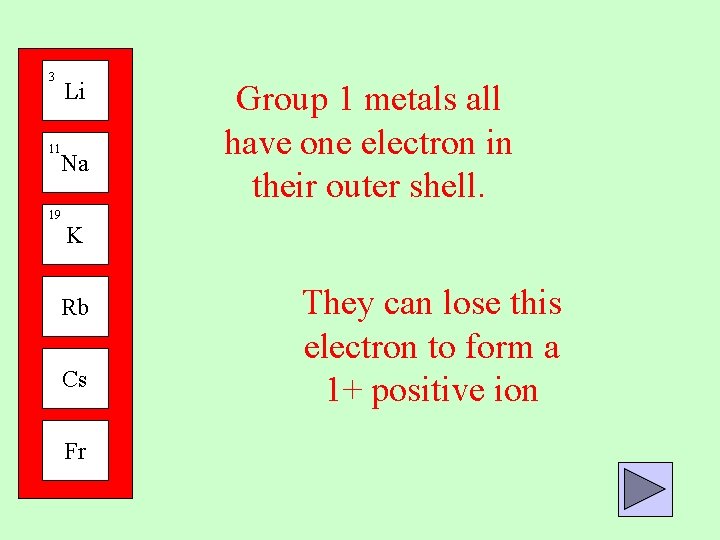
3 Li 11 Na 19 Group 1 metals all have one electron in their outer shell. K Rb Cs Fr They can lose this electron to form a 1+ positive ion
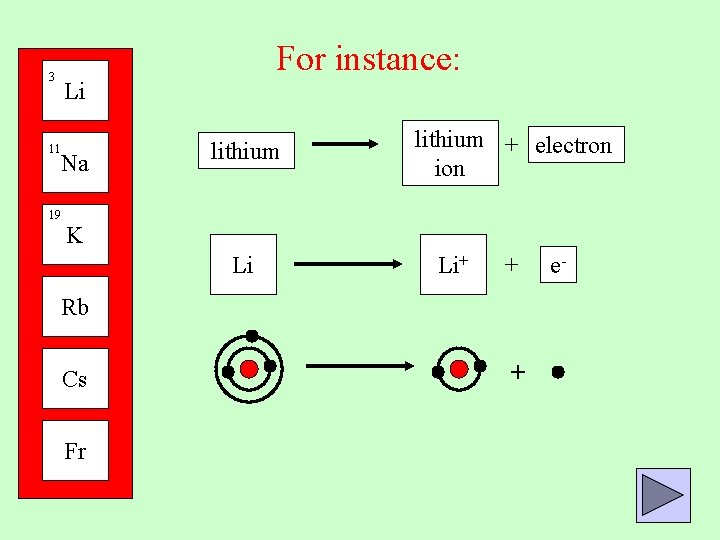
3 For instance: Li 11 Na 19 lithium + electron ion K Li Li+ + Rb Cs Fr + e-
3 Li 11 Na 19 K Rb Cs Fr + Li+
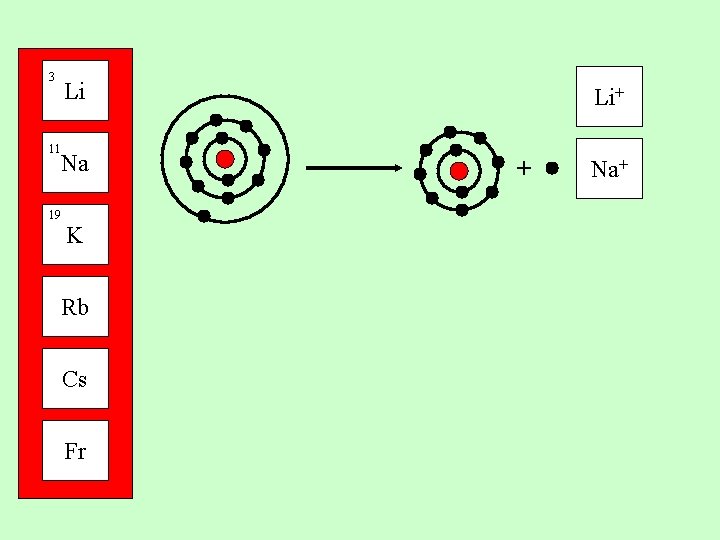
3 Li 11 Na 19 K Rb Cs Fr Li+ + Na+
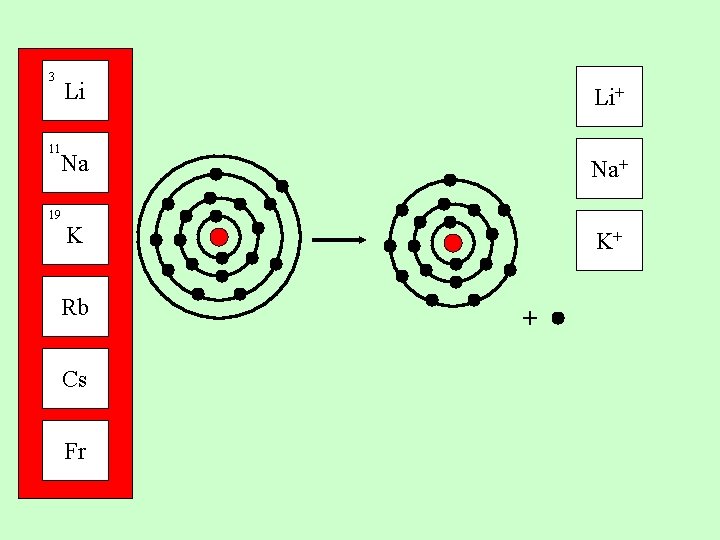
3 Li Li+ Na Na+ 11 19 K Rb Cs Fr K+ +
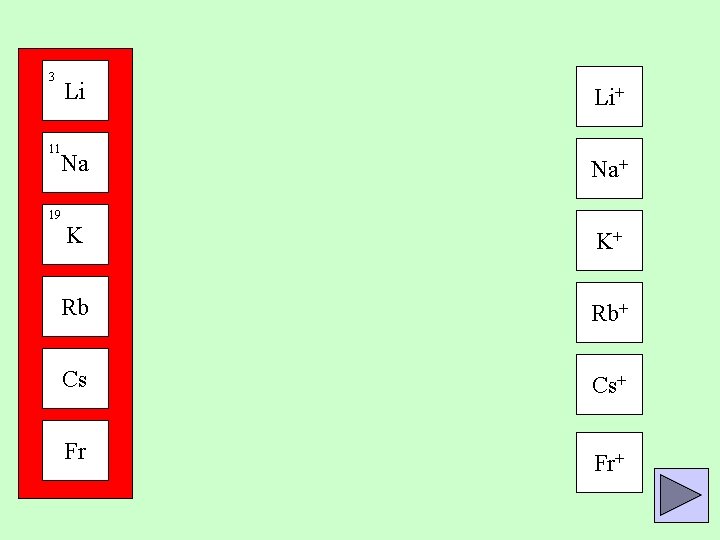
3 Li Li+ Na Na+ 11 19 K K+ Rb Rb+ Cs Cs+ Fr Fr+
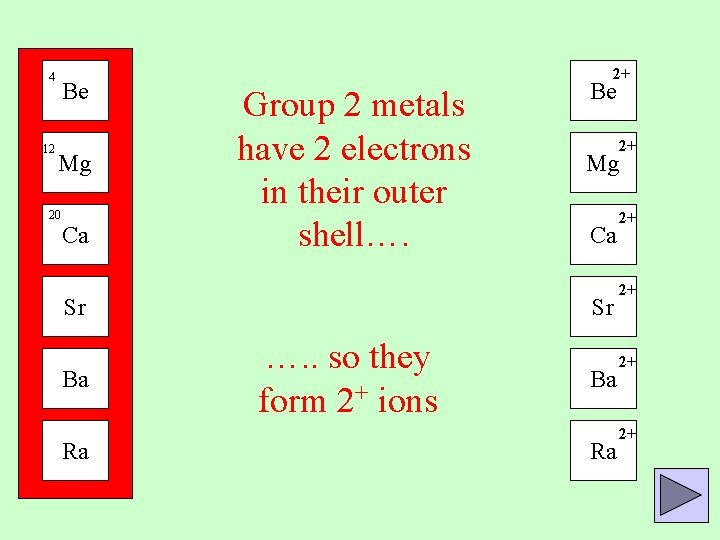
4 12 Be Mg 20 Ca 2+ Group 2 metals have 2 electrons in their outer shell…. Sr Ba Ra Be 2+ Mg Ca Sr …. . so they form 2+ ions Ba Ra 2+ 2+
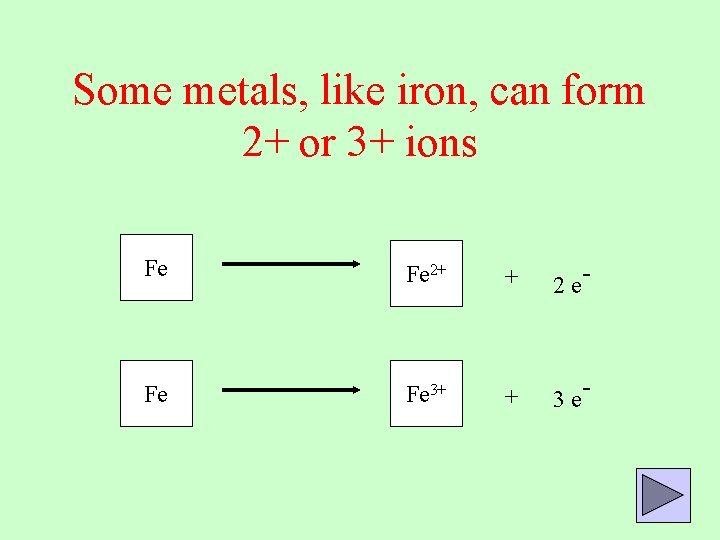
Some metals, like iron, can form 2+ or 3+ ions Fe Fe 2+ + 2 e- Fe Fe 3+ + 3 e-

Non-metals can form two types of ion: Monatomic ions Polyatomic ions (the ion is made from just one non-metal element) (the ion is made from more than one non-metal element) Cl - = chloride I - = iodide Br - = bromide OH- = hydroxide SO 42 NO - = sulphate O 2 - = oxide S 2 - = sulphide CO 32 - = carbonate HCO 2 - = hydrogen carbonate 3 3 = nitrate
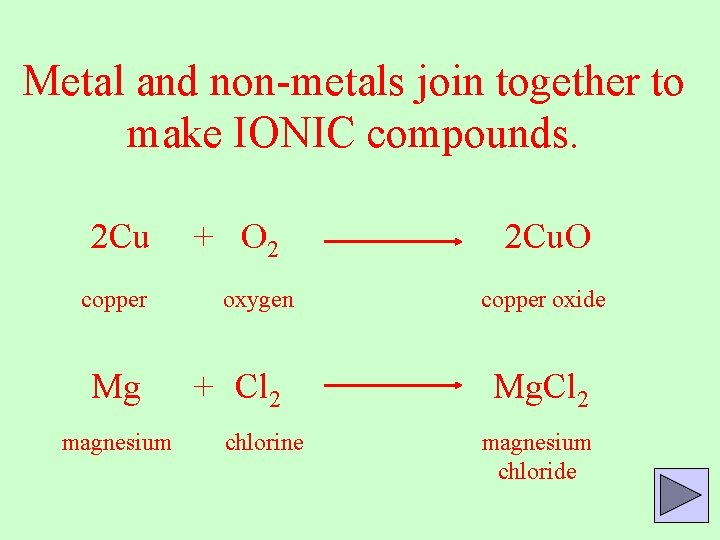
Metal and non-metals join together to make IONIC compounds. 2 Cu copper Mg magnesium + O 2 oxygen + Cl 2 chlorine 2 Cu. O copper oxide Mg. Cl 2 magnesium chloride
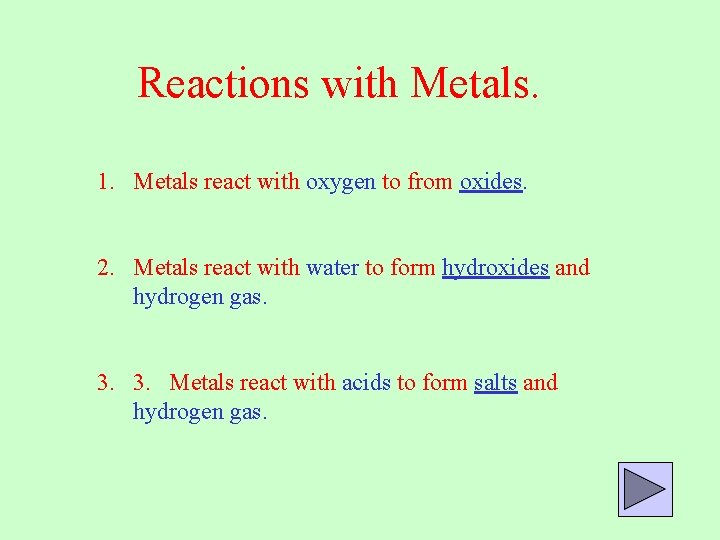
Reactions with Metals. 1. Metals react with oxygen to from oxides. 2. Metals react with water to form hydroxides and hydrogen gas. 3. 3. Metals react with acids to form salts and hydrogen gas.
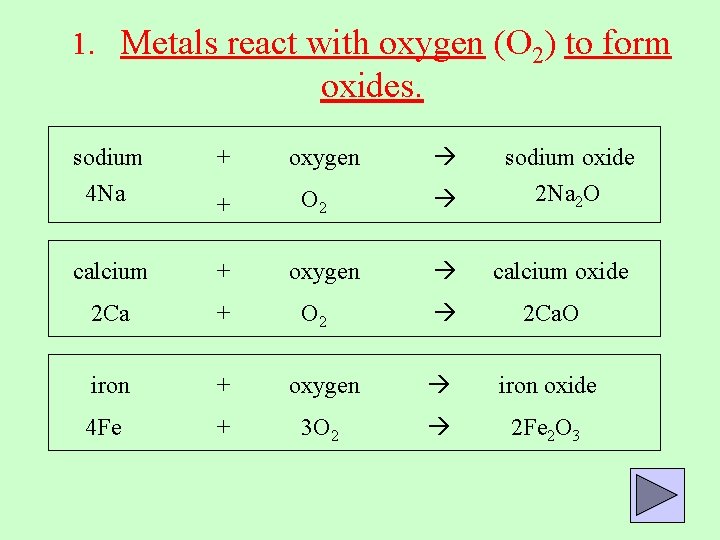
1. Metals react with oxygen (O 2) to form oxides. sodium + 4 Na + calcium + 2 Ca + iron + 4 Fe + oxygen O 2 oxygen sodium oxide 2 Na 2 O calcium oxide 2 Ca. O oxygen iron oxide 3 O 2 2 Fe 2 O 3 O 2
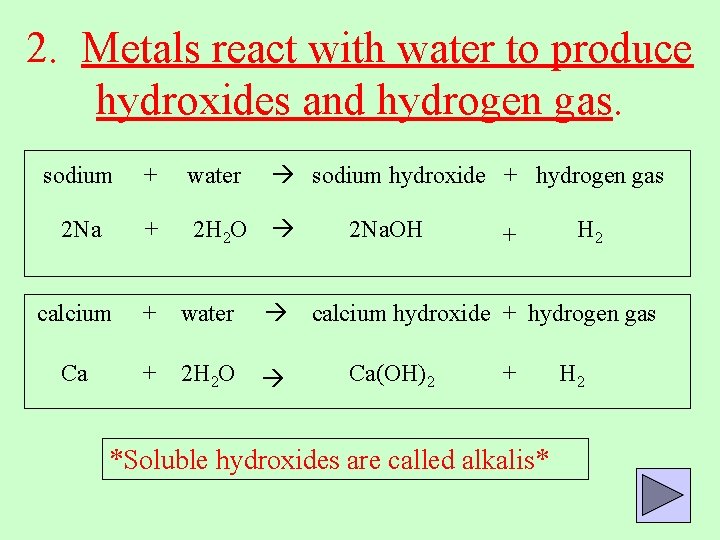
2. Metals react with water to produce hydroxides and hydrogen gas. sodium hydroxide + hydrogen gas sodium + water 2 Na + 2 H 2 O calcium + water Ca + 2 H 2 O 2 Na. OH + H 2 calcium hydroxide + hydrogen gas Ca(OH)2 + *Soluble hydroxides are called alkalis* H 2
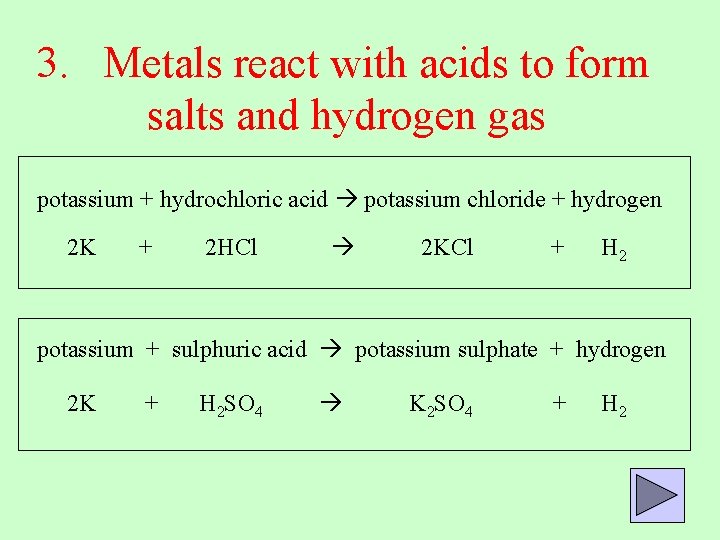
3. Metals react with acids to form salts and hydrogen gas potassium + hydrochloric acid potassium chloride + hydrogen 2 K + 2 HCl 2 KCl + H 2 potassium + sulphuric acid potassium sulphate + hydrogen 2 K + H 2 SO 4 K 2 SO 4 + H 2
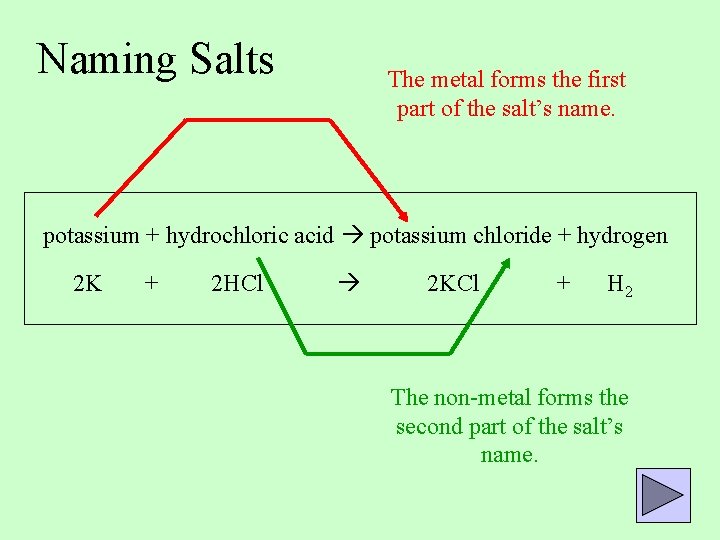
Naming Salts The metal forms the first part of the salt’s name. potassium + hydrochloric acid potassium chloride + hydrogen 2 K + 2 HCl 2 KCl + H 2 The non-metal forms the second part of the salt’s name.
- Slides: 41