Metals and Alloys CH 1121 Solids Two types

















- Slides: 35

Metals and Alloys CH 1121

Solids � Two types of solids ◦ Crystalline (faces, regular shapes) �Sodium chloride, quartz, diamond ◦ Amorphous (lack order) �Rubber, glass � Crystalline ◦ ◦ solids can be Ionic Molecular Covalent network Metallic solids

Crystalline Solids (Ionic) � Held together by mutual electrostatic attraction between cations and anions � Do not conduct electricity well � Brittle

Crystalline Solids (Molecular) � Held together by (relatively weak) intermolecular forces ◦ London dispersion ◦ Dipole-dipole ◦ Hydrogen bonding � Low � Soft Melting points

Crystalline Solids (Covalent Network) � Held together by extended network of covalent bonds � Results in materials that are extremely hard (diamond) � Semiconductors

Crystalline Solids (Metallic) � Held together by delocalized “sea” of electrons � Conduct electricity ◦ Electrons flow � Strong, not brittle

Network Solids � Three important network solids ◦ Diamond ◦ Graphite ◦ Silica

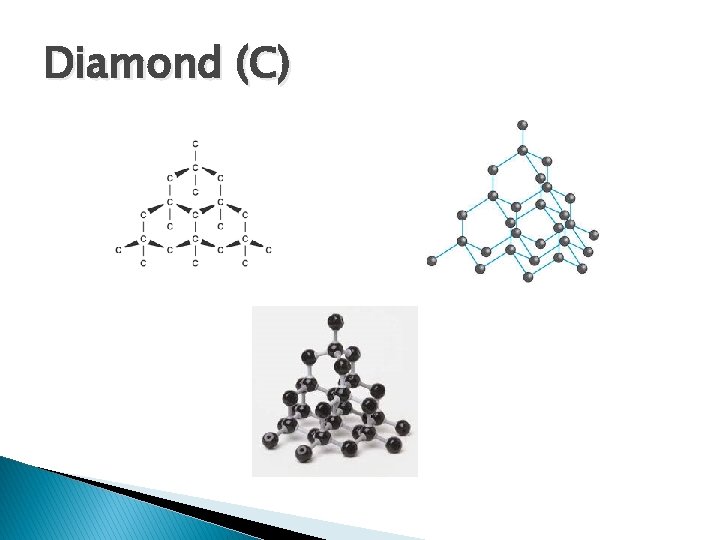
Diamond (C)

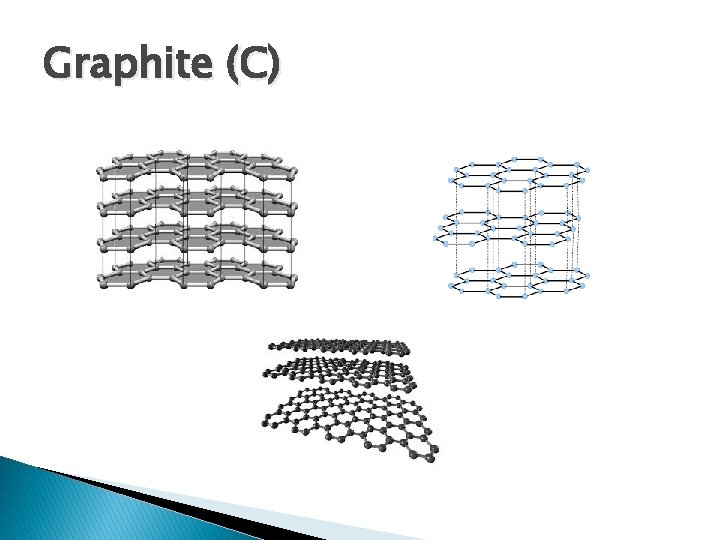
Graphite (C)

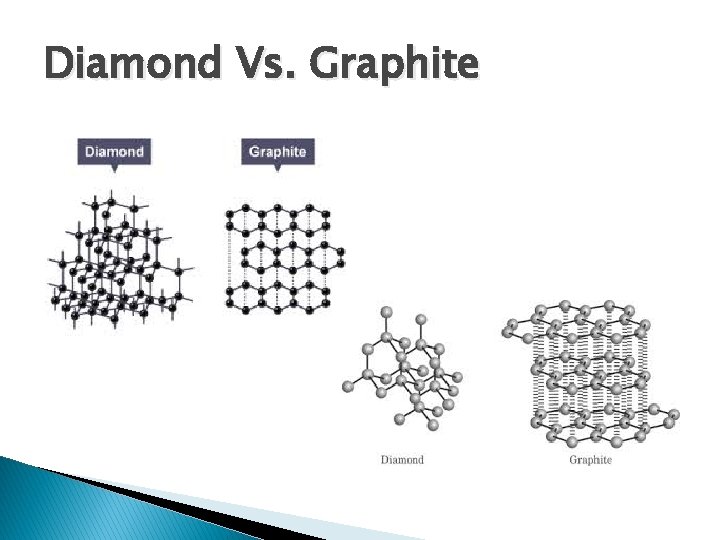
Diamond Vs. Graphite � Both are made of carbon atoms only � Diamond is stronger ◦ Made of many covalent bonds between carbon atoms � Graphite is weaker ◦ Made in sheets of covalent bonds with only weak intermolecular forces holding these sheets together

Diamond Vs. Graphite

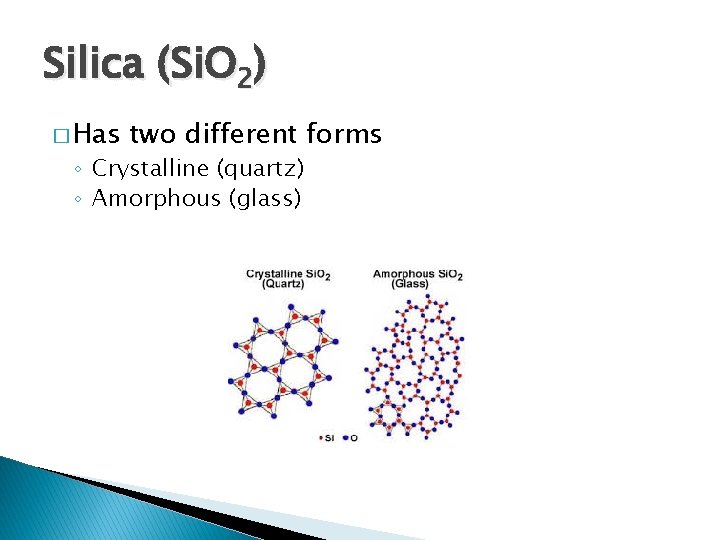
Silica (Si. O 2) � Has two different forms ◦ Crystalline (quartz) ◦ Amorphous (glass)

Silica (Si. O 2) � Crystalline (quartz) � Amorphous (glass)

Glass � Colour can be changed by adding metals and metal oxides ◦ ◦ ◦ ◦ Green = chromium Yellow, brown, black = carbon, iron, sulfur Purple = manganese (IV) oxide Blue-green = iron (II) oxide Blue = cobalt Red/pink = selenium White = tin, arsenic, antimony oxides

Glass

Glass � We can add substances to strengthen glass ◦ Mg. O (containers) ◦ Al 2 O 3 (Pyrex) ◦ Dipping in hot potassium salt solution

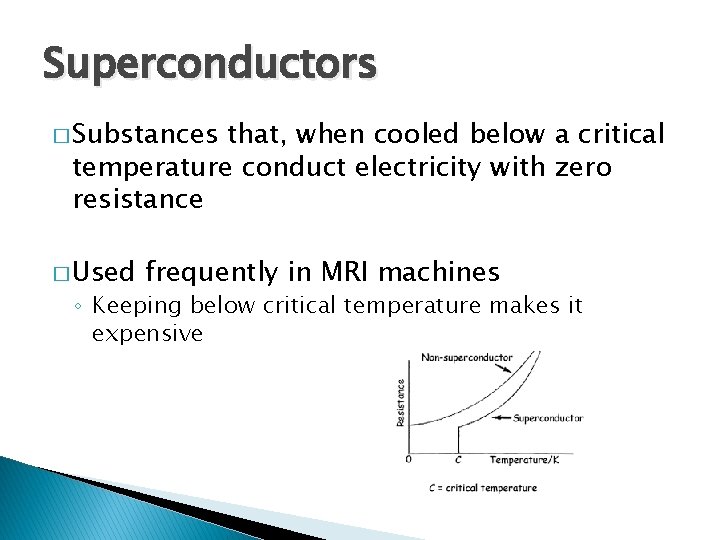
Superconductors � Substances that, when cooled below a critical temperature conduct electricity with zero resistance � Used frequently in MRI machines ◦ Keeping below critical temperature makes it expensive

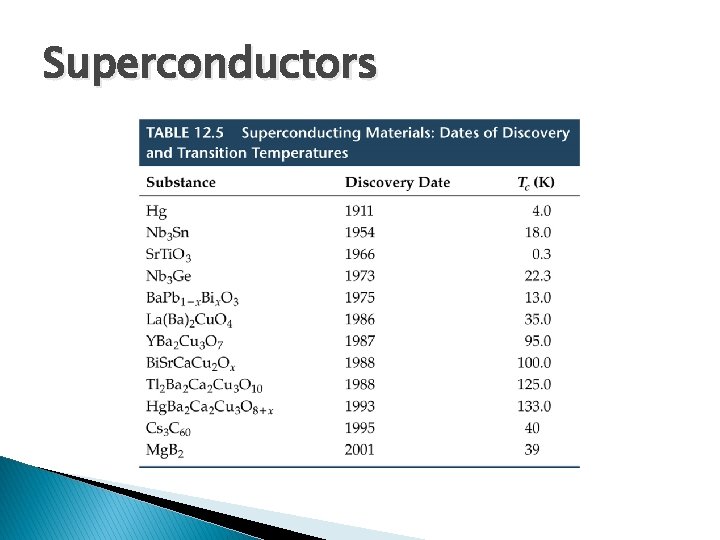
Superconductors

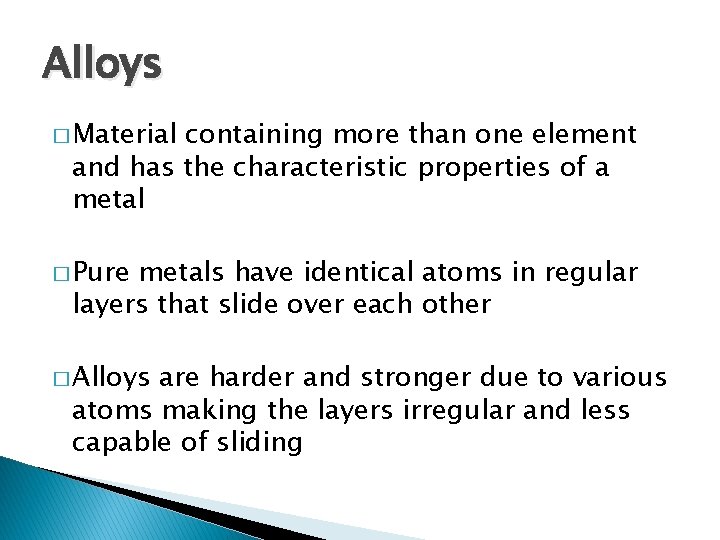
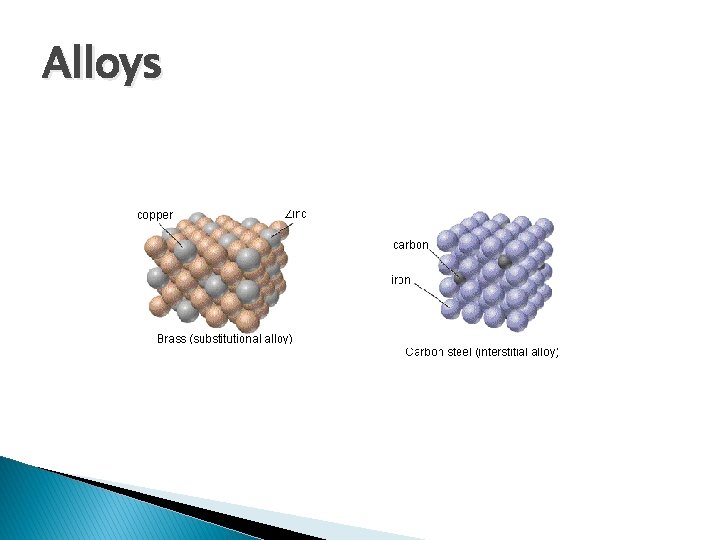
Alloys � Material containing more than one element and has the characteristic properties of a metal � Pure metals have identical atoms in regular layers that slide over each other � Alloys are harder and stronger due to various atoms making the layers irregular and less capable of sliding

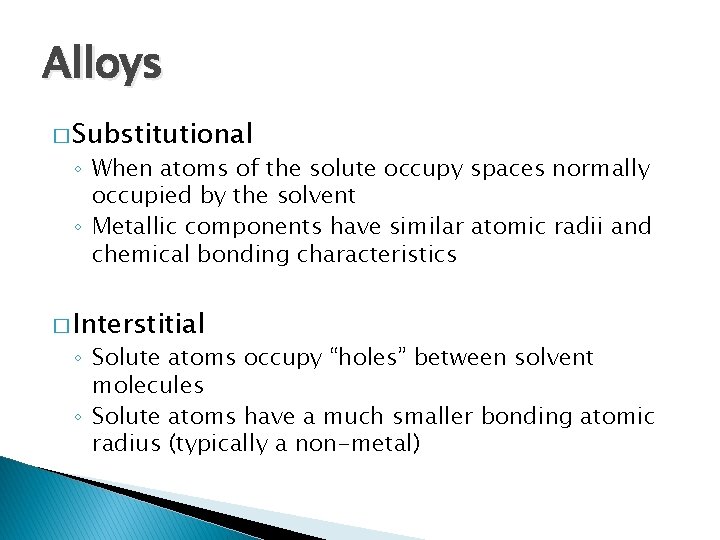
Alloys � Substitutional ◦ When atoms of the solute occupy spaces normally occupied by the solvent ◦ Metallic components have similar atomic radii and chemical bonding characteristics � Interstitial ◦ Solute atoms occupy “holes” between solvent molecules ◦ Solute atoms have a much smaller bonding atomic radius (typically a non-metal)

Alloys

Iron � Makes up 5% of Earth’s crust and the majority of Earth’s core � Pure iron is not strong enough to be used in the industrial setting, also in nature it rusts

Corrosion of Iron �

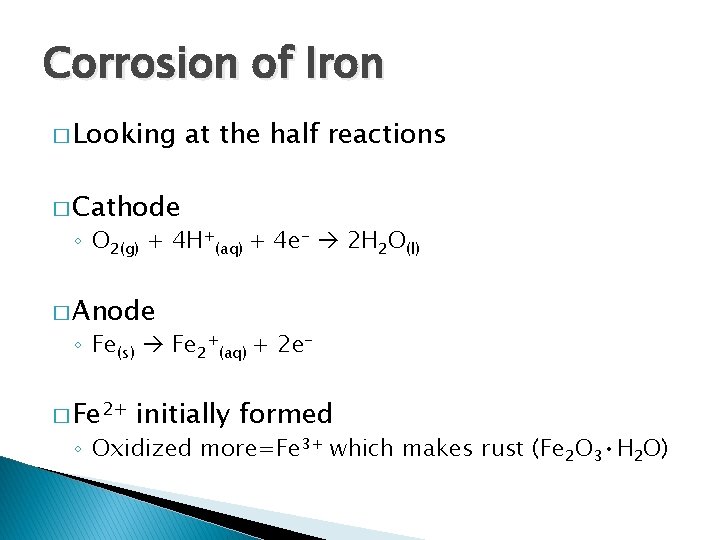
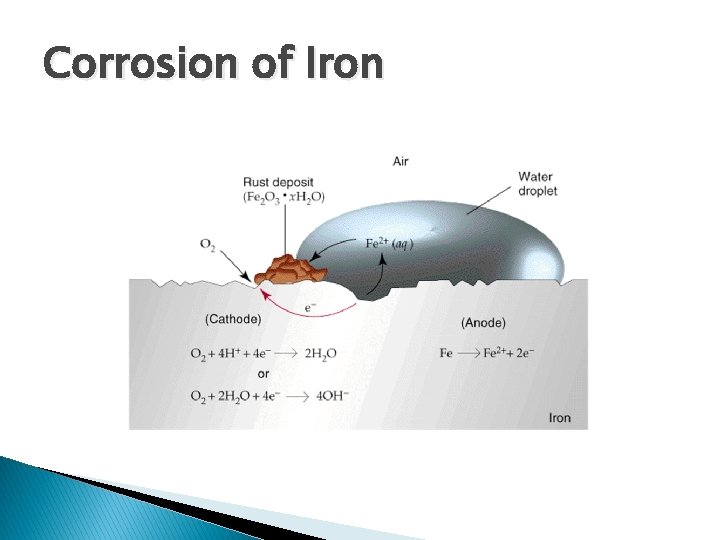
Corrosion of Iron � Looking at the half reactions � Cathode ◦ O 2(g) + 4 H+(aq) + 4 e- 2 H 2 O(l) � Anode ◦ Fe(s) Fe 2+(aq) + 2 e- � Fe 2+ initially formed ◦ Oxidized more=Fe 3+ which makes rust (Fe 2 O 3 • H 2 O)

Corrosion of Iron

Corrosion of Iron � Increasing ◦ ◦ ◦ Increased Increased rate of corrosion of metals presence of oxygen temperature presence of chemical salts humidity pollutants

Corrosion of Iron � Inhibiting corrosion ◦ Coat in paint or another metal (galvanized = Zn) ◦ Create alloy (steel) ◦ Cathodic protection (make iron the cathode, attach a sacrificial metal)

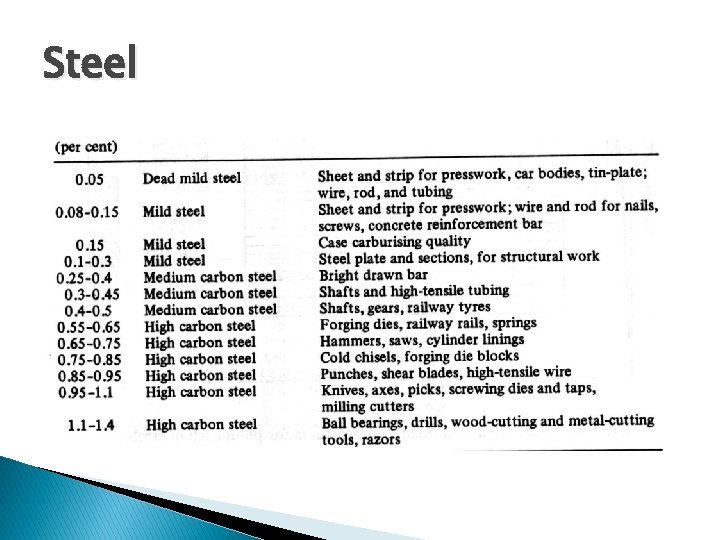
Steel � Alloy of iron and carbon � Carbon steel contains just iron and carbon � Alloy steel contains iron, carbon, and other elements ◦ Sulfur makes steel lose strength at high temperatures ◦ Copper and nickel increase strength of steel

Steel � Stainless steel is alloy steel with chromium, nickel, or both ◦ More corrosion resistant and harder than carbon steel � Chromium hardened � Nickel stainless steel is brighter, can be stainless steel is more corrosion resistant

Steel

Aluminum � Useful ◦ ◦ as an engineering material Lightweight Abundant in nature Resists corrosion Good conductor � Uses of alloys ◦ With copper and magnesium for aircraft parts ◦ Increase strength of bronzes

Copper, Brass, and Bronze � Brass ◦ Copper and Zinc ◦ Plumbing, decoration � Bronze ◦ Copper and any other metal ◦ Marine hardware, jewelry � Copper alloys are useful for when heat needs to be dissipated

Copper � Copper has high conductivity � Must be pure in electrical applications because any impurities would compromise effectiveness ◦ Add resistance ◦ Cause wiring to heat up

White Metals � Lead ◦ ◦ ◦ High density High ductility High corrosion resistance Low melting point Toxic Lead-acid storage batteries, pipes, radiation shield, with tin in solder

White Metals � Tin ◦ Low toxicity ◦ Food applications (tin cans), pewter, with other metals in solder � Zinc ◦ Galvanizing steel, sacrificial anodes, battery casings in dry cell and alkaline batteries, brass