Metallurgy Crystal Structure Chapter 7 Learning Objectives Explain

Metallurgy Crystal Structure Chapter 7

Learning Objectives Explain how a crystal is formed in metal Discuss the formation of space lattice structures Describe the appearance of atoms inside crystals Explain how temperature affects the growth of a crystal Tell what is meant by grain size.

Crystals Iron and Steel melt into liquid form at high temperatures. When liquid gradually cools, crystals slowly begin to form and the steel solidifies. Tiny crystals from first. They keep growing until lined up neatly and precisely together.

Space Lattice The organized arrangement of atoms in a crystal is called the space lattice. Not all metals have the same pattern of atoms. Most fundamental arrangement is the Unit cell.

Unit Cells Space lattice is a group of unit cells that are identical. 1. atoms make up unit cells 2. unit cells make up a space lattice 3. space lattices make up a crystal

Unit Cells 4 most common unit cells Body-centered cubic Face –centered cubic Close-packed hexagonal Body-centered tetragonal Fig 7 -3 Page 120

Body Centered Cubic • Cell has a cubic shape • Atoms at 8 corners with 9 th atom in the center. Metals that commonly have Body Centered Cubic Formation • Chromium • Molybdenum • Tantalum • Tungsten • Vanadium • Niobium • and the Ferrite form of Iron.

Face Centered Cubic • Cell has a cubic shape • Atoms at 8 corners with 1 atom in the center of each of the six faces. Metals that commonly have Face Centered Cubic Formation • Aluminum • Copper • Gold • Lead • Nickel • Platinum • Silver • Austenitic Iron

Close-Packed Hexagonal • 17 Atoms • Very brittle shape found in metals that have little ductility. Metals that commonly have Close-packed Hexagonal Formation • Cadmium • Cobalt • Magnesium • Titanium • Beryllium • Zinc

Body-Centered Tetragonal • 9 Atoms • Similar to body-centered cubic • Faces rectangular instead of square. • Strongest, Hardest and most Brittle • Only Ferrous metal to make this formation is Martensitic Iron.
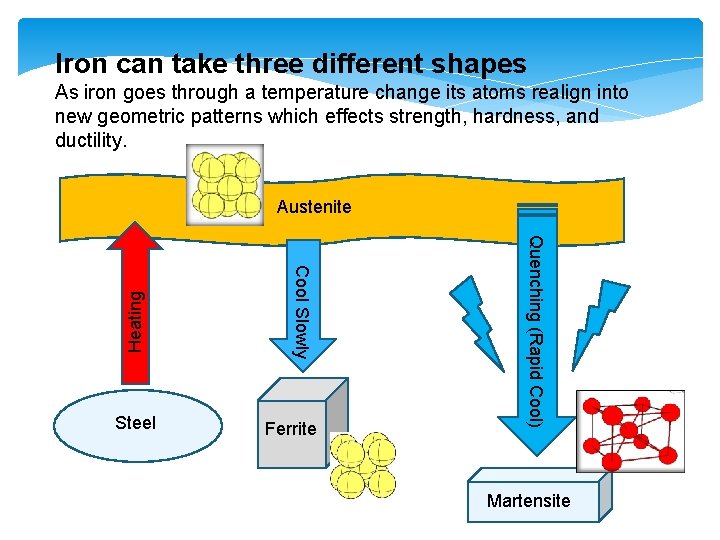
Iron can take three different shapes As iron goes through a temperature change its atoms realign into new geometric patterns which effects strength, hardness, and ductility. Ferrite Quenching (Rapid Cool) Steel Cool Slowly Heating Austenite Martensite
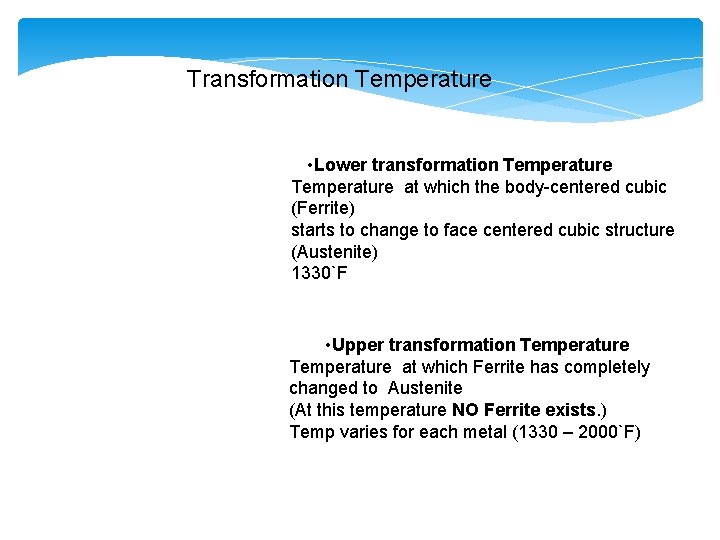
Transformation Temperature • Lower transformation Temperature at which the body-centered cubic (Ferrite) starts to change to face centered cubic structure (Austenite) 1330`F • Upper transformation Temperature at which Ferrite has completely changed to Austenite (At this temperature NO Ferrite exists. ) Temp varies for each metal (1330 – 2000`F)
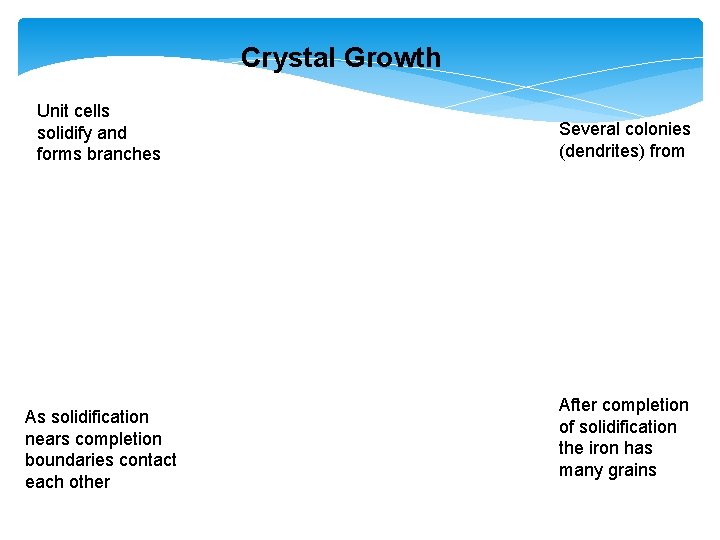
Crystal Growth Unit cells solidify and forms branches As solidification nears completion boundaries contact each other Several colonies (dendrites) from After completion of solidification the iron has many grains
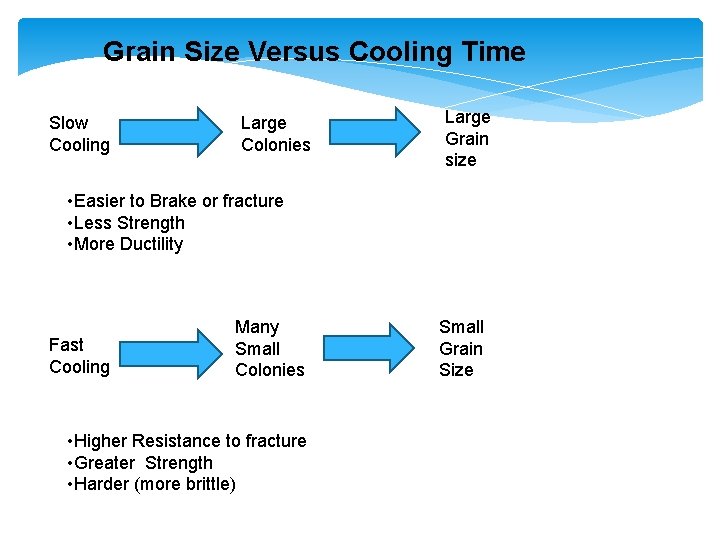
Grain Size Versus Cooling Time Slow Cooling Large Colonies Large Grain size • Easier to Brake or fracture • Less Strength • More Ductility Fast Cooling Many Small Colonies • Higher Resistance to fracture • Greater Strength • Harder (more brittle) Small Grain Size
- Slides: 15