METALLIC POLAR COVALENT BONDS DESCRIBE HOW METALS BOND
METALLIC & POLAR COVALENT BONDS DESCRIBE HOW METALS BOND AND FORM ALLOYS DESCRIBE THE WAY DIFFERENT MOLECULES BOND TO EACH OTHER
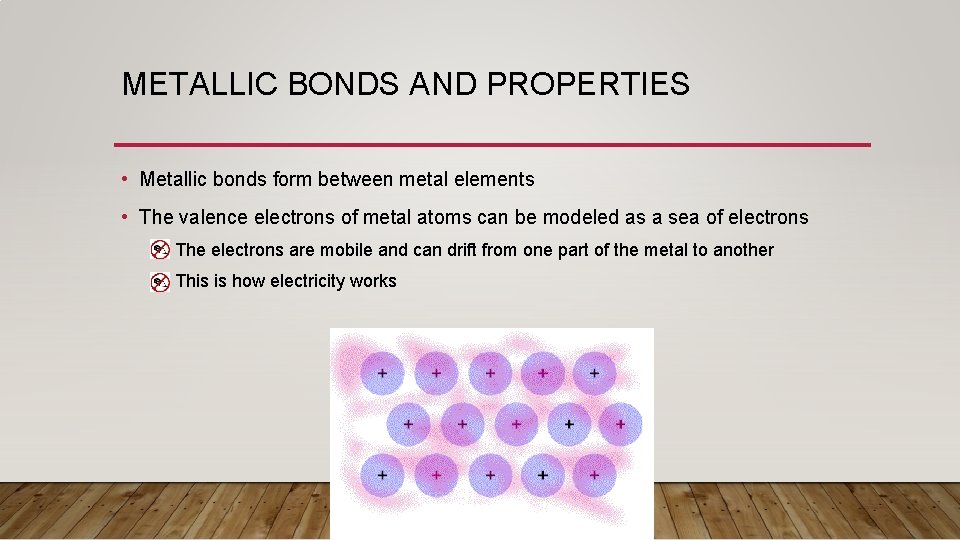
METALLIC BONDS AND PROPERTIES • Metallic bonds form between metal elements • The valence electrons of metal atoms can be modeled as a sea of electrons • The electrons are mobile and can drift from one part of the metal to another • This is how electricity works
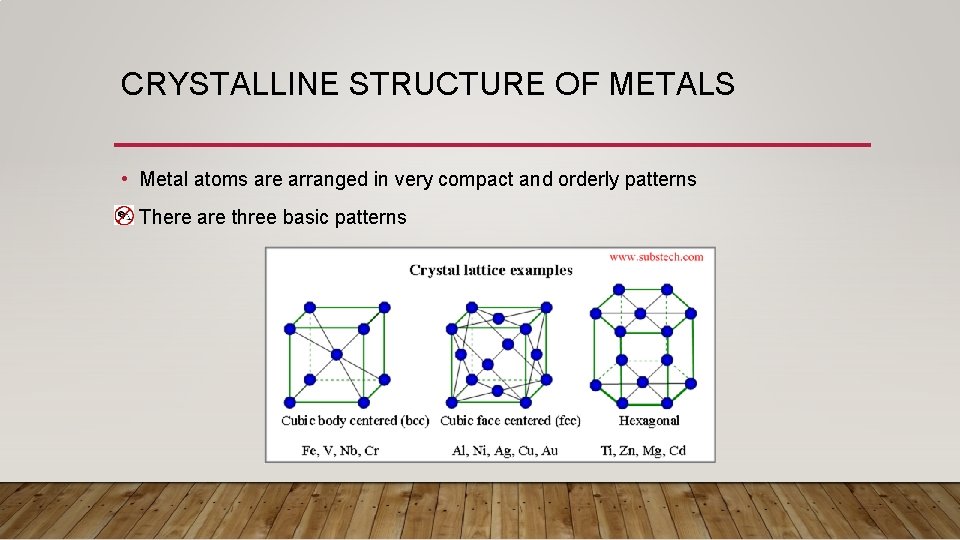
CRYSTALLINE STRUCTURE OF METALS • Metal atoms are arranged in very compact and orderly patterns • There are three basic patterns

ALLOYS • The properties of alloys are often better than those of their component elements • Examples: • Sterling silver (silver and copper) is more durable than pure silver • Steels (iron, carbon, boron, chromium etc) resist corrosion, have better ductility, hardness, and toughness
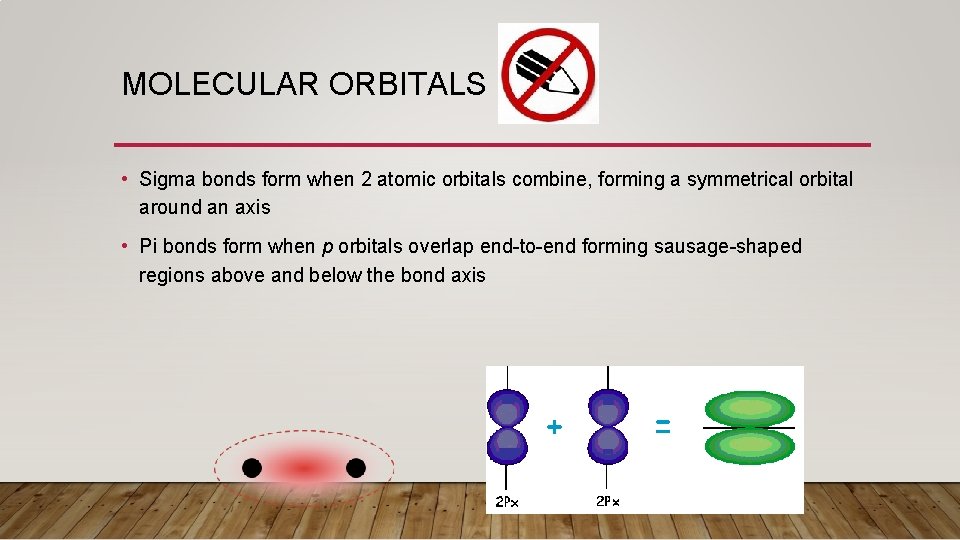
MOLECULAR ORBITALS • Sigma bonds form when 2 atomic orbitals combine, forming a symmetrical orbital around an axis • Pi bonds form when p orbitals overlap end-to-end forming sausage-shaped regions above and below the bond axis
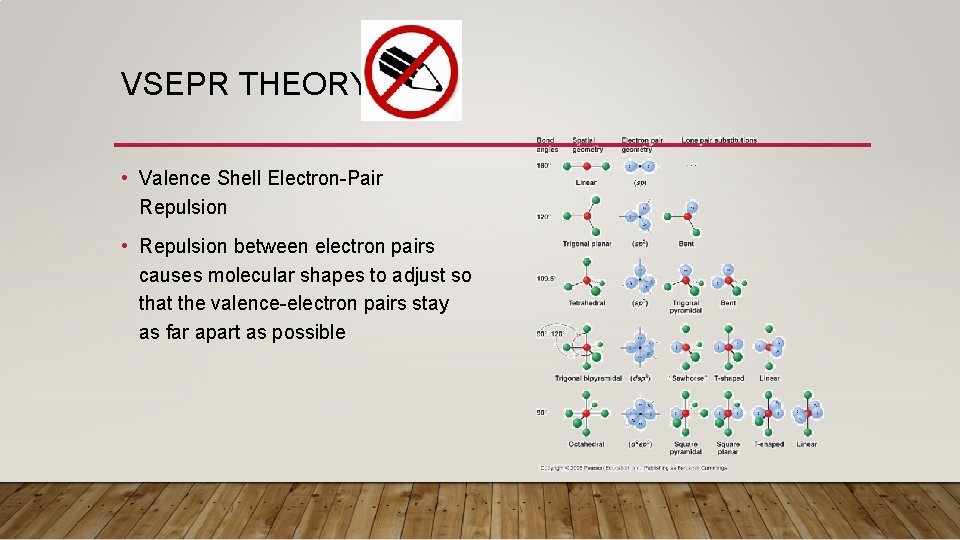
VSEPR THEORY • Valence Shell Electron-Pair Repulsion • Repulsion between electron pairs causes molecular shapes to adjust so that the valence-electron pairs stay as far apart as possible
BOND POLARITY • Nonpolar covalent bonds form when bonding electrons are shared equally • Diatomic molecules are nonpolar • Polar covalent bond, or a polar bond, is a bond between atoms in which the electrons are shared unequally • The more electronegative atom attracts electrons and gains a slight negative charge, the less electronegative atom gains a slightly positive charge. • Example: Water
ATTRACTIONS BETWEEN MOLECULES • Van der Waals Forces consist of dipole interactions and dispersion forces • Weakest attractions between molecules • Dipole interactions occur when polar molecules are attracted to one another • Dispersion forces occur when moving electrons in a nonpolar molecule are momentarily more on one side than other. Momentary dipole
HYDROGEN BONDS • Attractive forces in which a hydrogen which is covalently bonded to a very electronegative atom is also weakly bonded to an unshared electron pair of another electronegative atom.

NETWORK SOLID • A solid in which all of the atoms are covalently bonded to each other • Examples: Diamond and Silicon carbide

IONIC VS COVALENT LAB HYPOTHESIS (IN LAB NOTEBOOK) • What properties of a compound will you use to determine if a bond is ionic or covalent? Choose three properties be sure to include why these properties will be useful in determining the bond type. • You will be writing a conclusion for the ionic vs covalent lab. You will be asked to provide multiple pieces of evidence for why you labeled a compound ionic or covalent. You will also be asked to include an evaluation of your hypothesis. Was your initial plan to test specific properties of a compound in support of your findings?
- Slides: 11