Metallic Bonds Metallic Bonds How can metals stick

Metallic Bonds

Metallic Bonds • How can metals stick together? § Ionic bonds? No, neither atom has a high electronegativity. § Covalent bonds? No, both atoms would have to have high electronegativities.

Metallic Bonds • All of the valence e− of metals are shared by all atoms—a grand sharing of outer e−. • This is called the free e− theory. • Almost all metal atoms have either one or two valence e− to be shared.
Types of Bonds 3 types 3. Metallic—two metals

Metallic Bonds • This explains many of the properties of metals. • Metallic bonds are defined as positive metal ions embedded in a sea of negative e−.

Metallic Bonds • A metallic bond achieves stability without directly satisfying the octet rule. • Atoms of different metals can bond to form an alloy. • Some alloys are true compounds, while some are heterogeneous mixtures.

Metallic Bonds • The hexagonal close packed (HCP) is the most efficient metal structure.
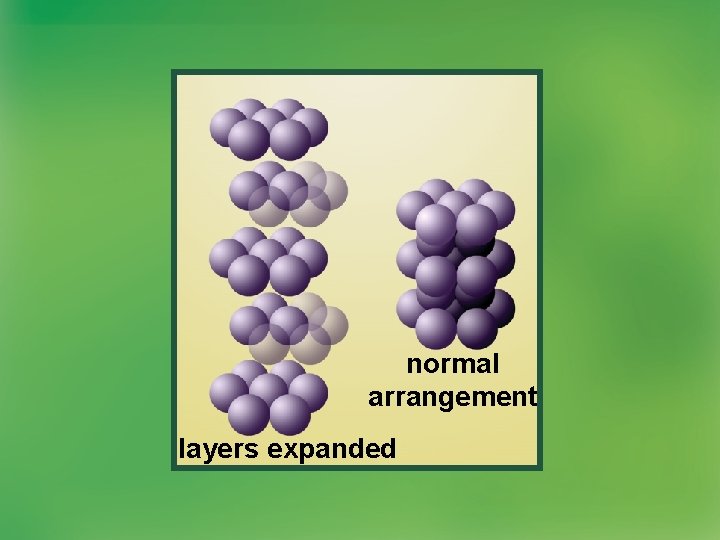
normal arrangement layers expanded

Properties of Metals • Conduct electricity § due to loose e− • Conduct heat § because of their free e− • Have a shiny luster § Loose electrons can easily jump up and down energy levels. § Remember Bohr and the emission of e− and colors.

Properties of Metals • Malleable § can be hammered into shapes • Ductile § can be drawn into wires
Types of Bonding Be sure to understand the differences between the three types of bonds presented in this chapter by learning the chart explaining the characteristics of bonding types (p. 432).

Which type of bond is found in a yellowish-white solid that has a high melting point and is soluble in water? 1. Covalent 2. Ionic 3. Metallic Question

Which type of bond is found in a reddish-brown liquid that is made up of molecules and does not conduct electricity? 1. Covalent 2. Ionic 3. Metallic Question

Which type of bond is found in a substance that has a silverywhite luster and is ductile, a good conductor of electricity, and insoluble in water? 1. Covalent 2. Ionic 3. Metallic Question
- Slides: 14