Metallic Bonds 2 a Students know atoms combine
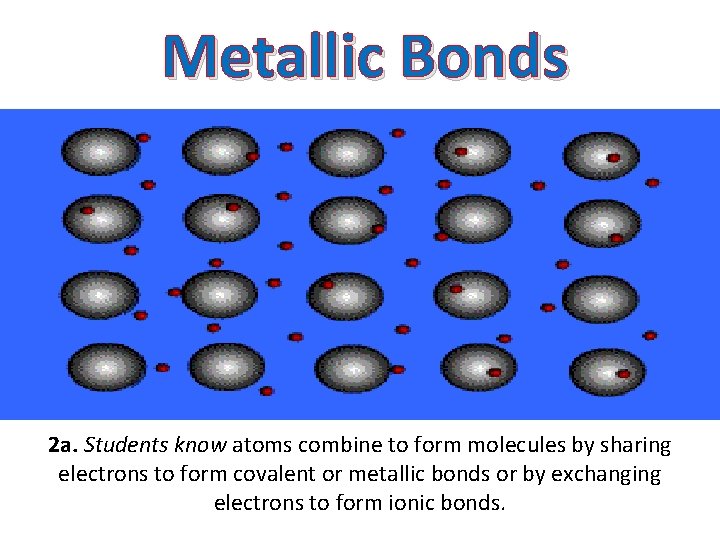
Metallic Bonds 2 a. Students know atoms combine to form molecules by sharing electrons to form covalent or metallic bonds or by exchanging electrons to form ionic bonds.
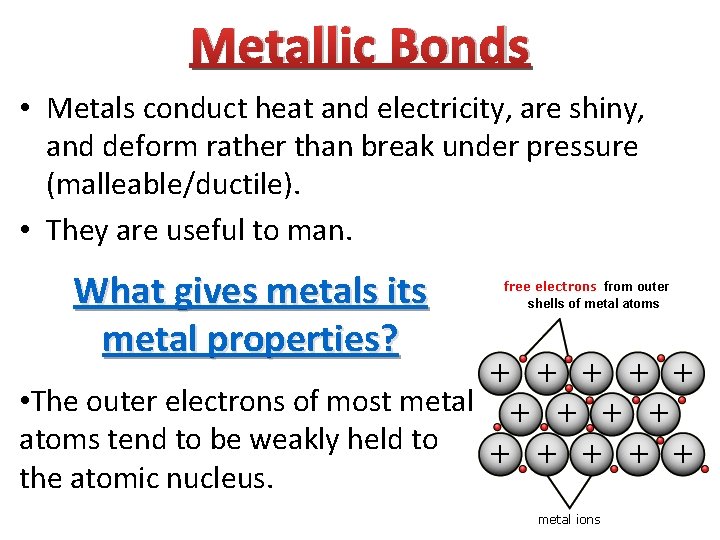
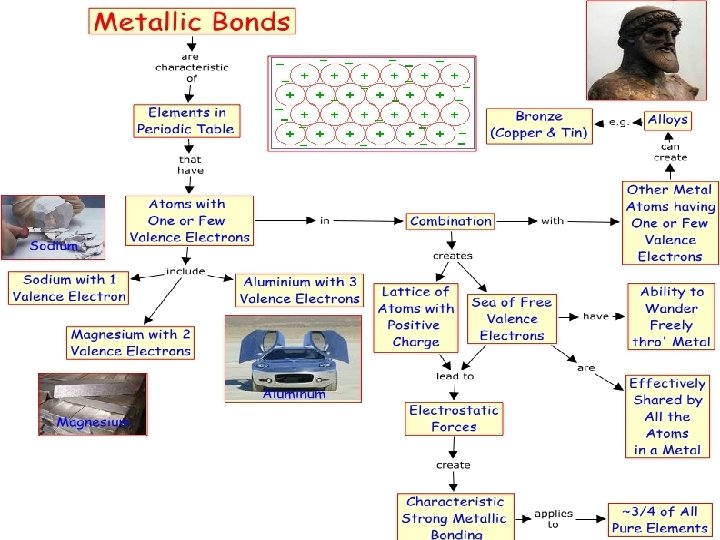
Metallic Bonds • Metals conduct heat and electricity, are shiny, and deform rather than break under pressure (malleable/ductile). • They are useful to man. What gives metals its metal properties? • The outer electrons of most metal atoms tend to be weakly held to the atomic nucleus.
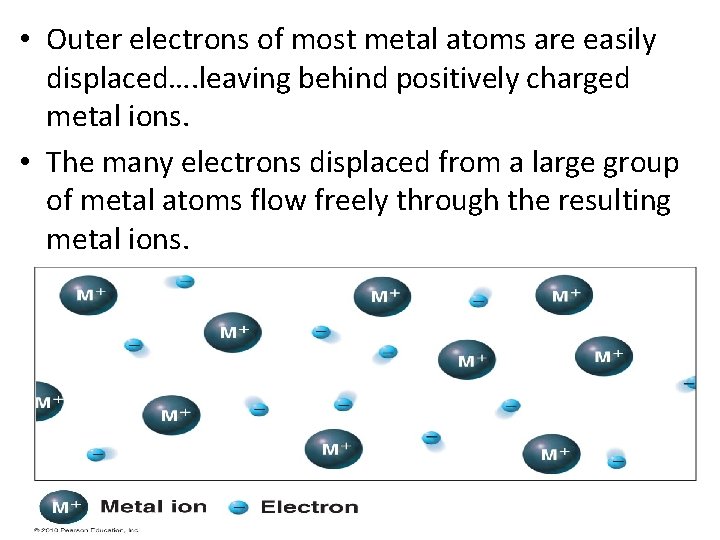
• Outer electrons of most metal atoms are easily displaced…. leaving behind positively charged metal ions. • The many electrons displaced from a large group of metal atoms flow freely through the resulting metal ions.

• This “fluid” of negatively charged electrons holds the positively charged metal ions together in the type of chemical bond known as a metallic bond. • The mobility of electrons in a metal accounts for the metal’s significant ability to conduct electricity and heat; gives them their shiny, opaque quality; and allows them to be molded into various shapes. Sea of electrons creates electricity!
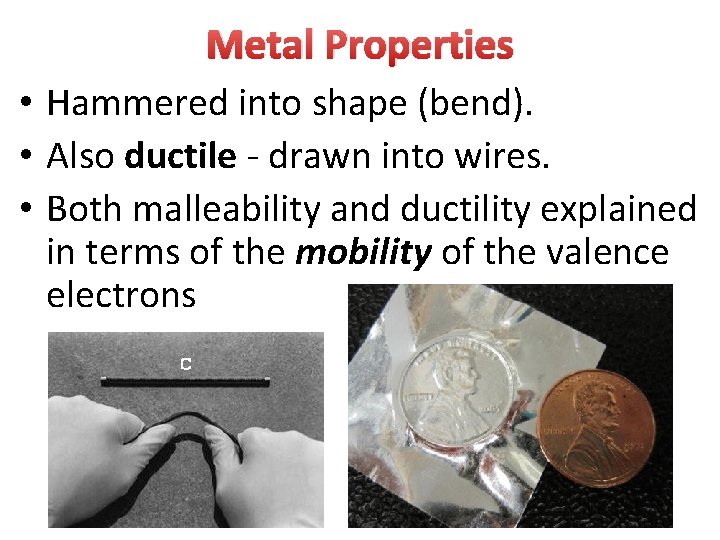
Metal Properties • Hammered into shape (bend). • Also ductile - drawn into wires. • Both malleability and ductility explained in terms of the mobility of the valence electrons

Due to the mobility of the valence electrons, metals have: 1) Ductility and 2) Malleability Notice that the ionic crystal breaks due to ion repulsion!
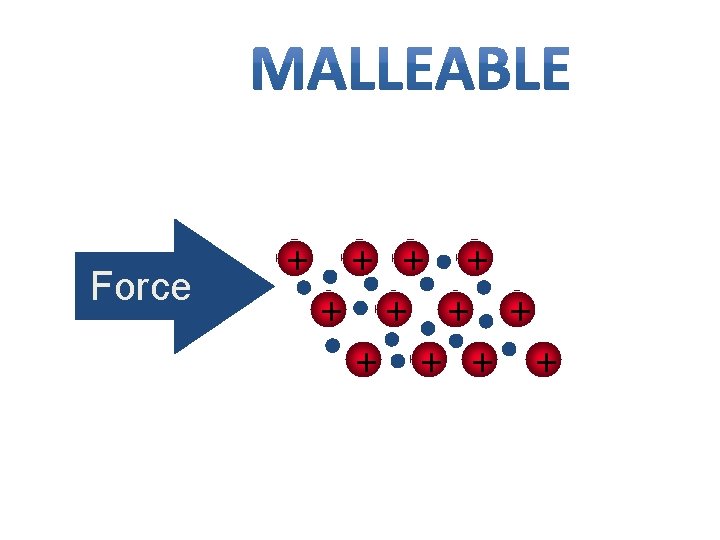
Force + + +
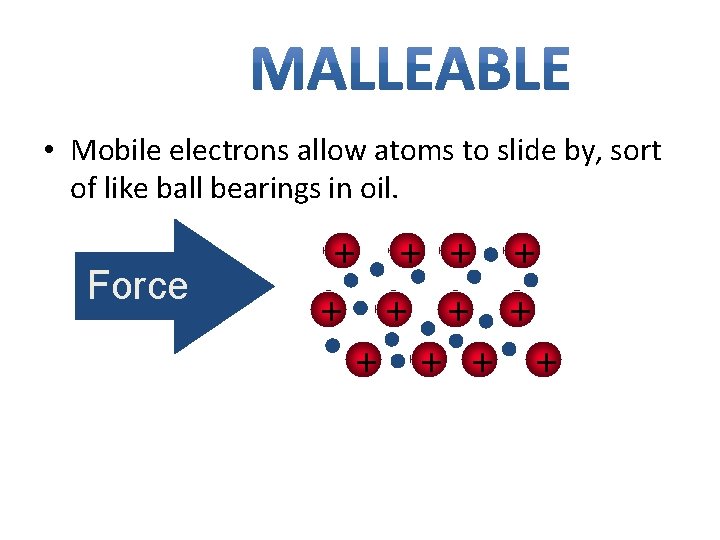
• Mobile electrons allow atoms to slide by, sort of like ball bearings in oil. Force + + +
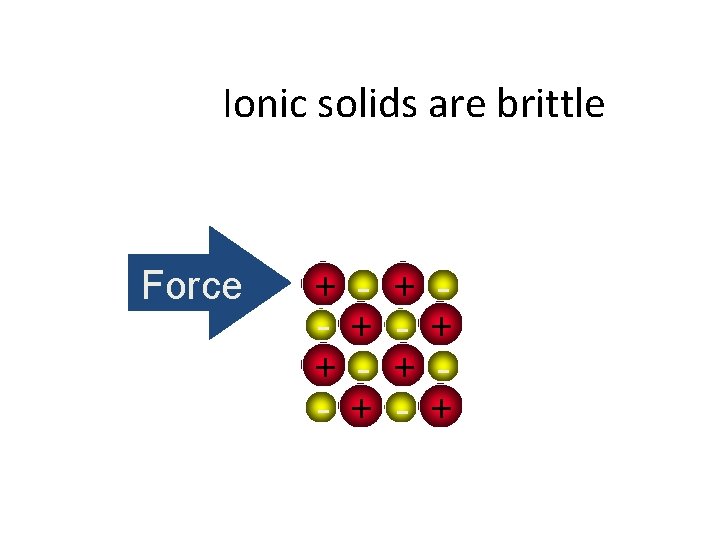
Ionic solids are brittle Force + + - + +

Ionic solids are brittle • Strong Repulsion breaks a crystal apart, due to similar ions being next to each other. Force + + - + - + - +

Alloys • We use lots of metals every day, but few are pure metals • Alloys are mixtures of 2 or more elements, at least 1 is a metal • made by melting a mixture of the ingredients, then cooling • Brass: an alloy of Cu and Zn • Bronze: Cu and Sn
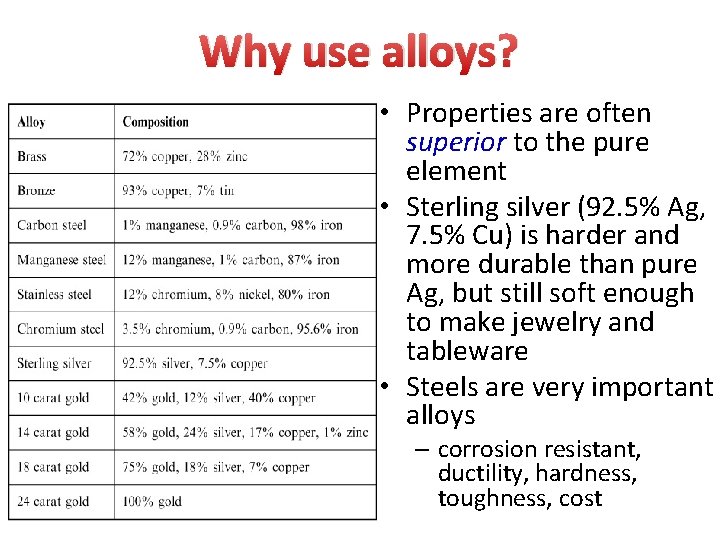
Why use alloys? • Properties are often superior to the pure element • Sterling silver (92. 5% Ag, 7. 5% Cu) is harder and more durable than pure Ag, but still soft enough to make jewelry and tableware • Steels are very important alloys – corrosion resistant, ductility, hardness, toughness, cost
- Slides: 13