Metallic Bonding Year 8 Extension Metals Structure of

Metallic Bonding Year 8 – Extension
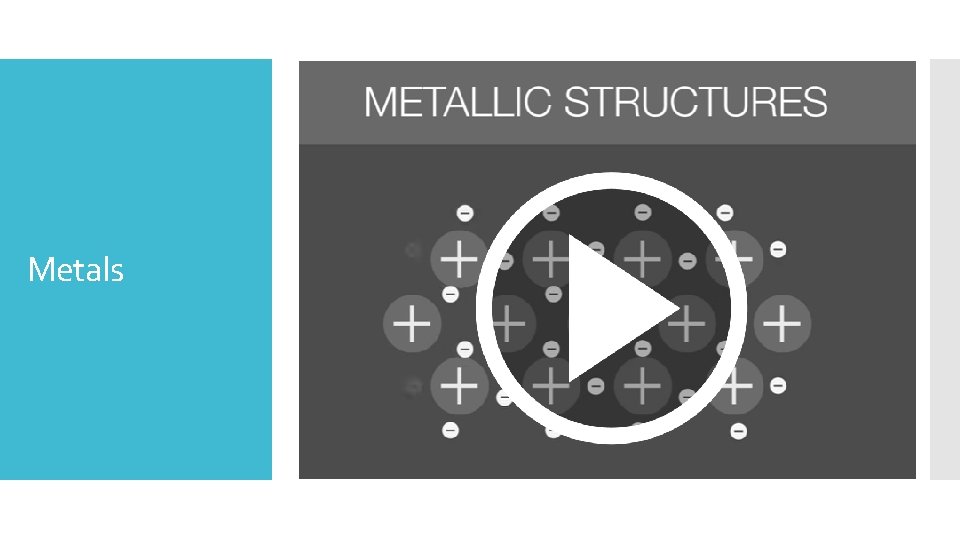
Metals

Structure of atoms within a metallic element Positive ions form the crystal lattice, while delocalised electrons move freely throughout the lattice, gluing the whole lattice together.

In the metallic bonding model, positive metal cations are surrounded by a sea of delocalised valence electrons Electrons are delocalised because they belong to the lattice as a whole rather than an individual atom Metallic Bonding Model Positively charged metal ions Delocalised sea of electrons
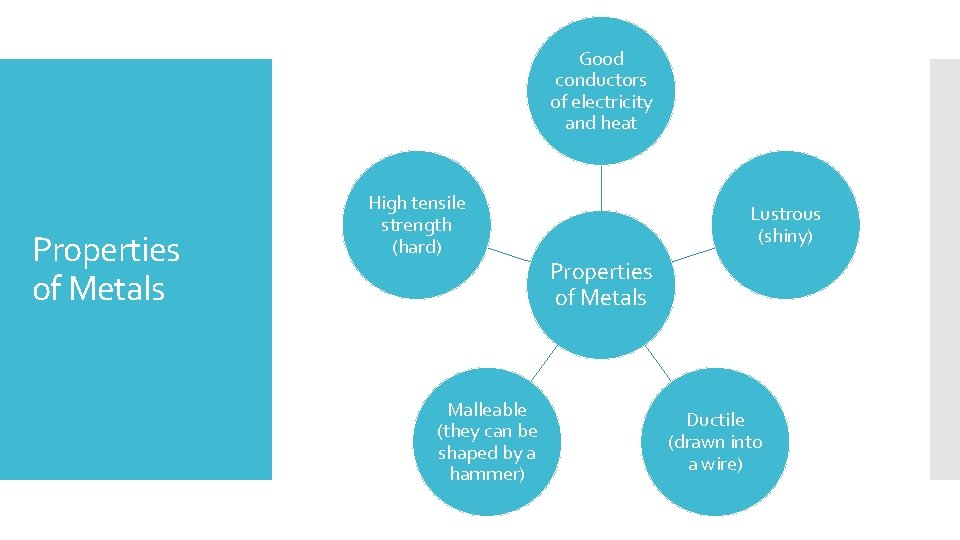
Good conductors of electricity and heat Properties of Metals High tensile strength (hard) Malleable (they can be shaped by a hammer) Lustrous (shiny) Properties of Metals Ductile (drawn into a wire)

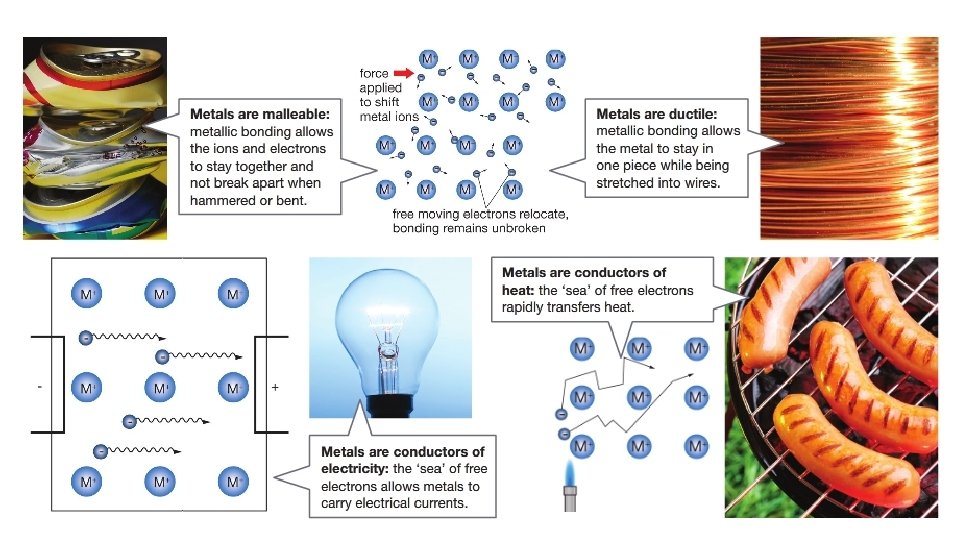
Strong electrostatic forces of attraction between positive metal ions and the sea of delocalised electrons holds the metallic lattice together Metals are hard and have relative high boiling points

Metals are Malleable and Ductile When a force is added, this causes metal ions to move past each other, layers of ions are still held together by the delocalised electrons between them

Free-moving delocalised electrons will move towards a positive electrode and away from a negative electrode in an electric circuit Metals are good conductors of electricity
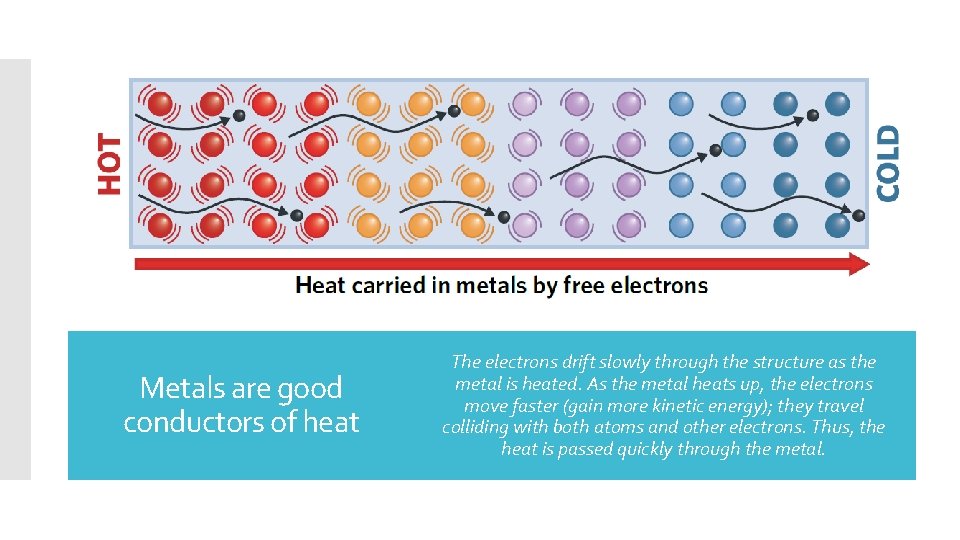
Metals are good conductors of heat The electrons drift slowly through the structure as the metal is heated. As the metal heats up, the electrons move faster (gain more kinetic energy); they travel colliding with both atoms and other electrons. Thus, the heat is passed quickly through the metal.
Properties of Metals
- Slides: 10