Metallic Bonding Metals in the periodic table Metals

Metallic Bonding

Metals in the periodic table
Metals Essential for Life Oops!

Properties of Metals • • • Solids (except Hg) – most have high mp & bp Luster Malleable & ductile Good conductor of heat & electricity Metals are losers! (metals lose electrons and form positive ions) • Low ionization energy • Low electronegativity

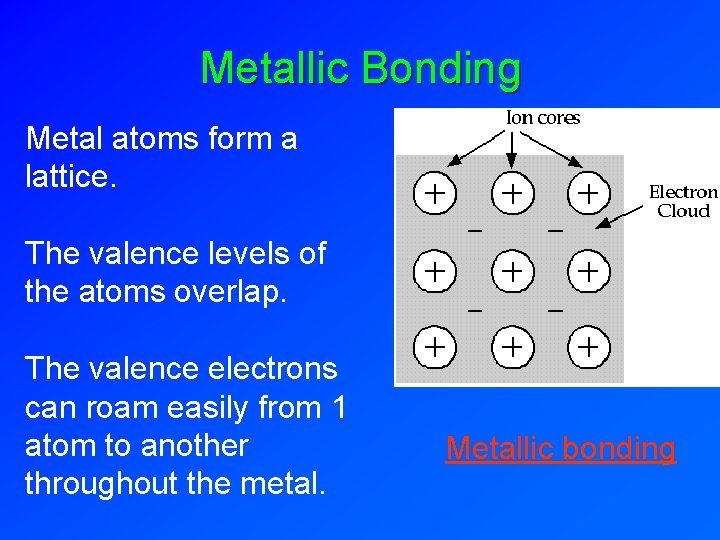
Metallic Bonding Metal atoms form a lattice. The valence levels of the atoms overlap. The valence electrons can roam easily from 1 atom to another throughout the metal. Metallic bonding

Delocalized Electrons • Valence electrons are free to roam throughout crystal • “Sea of Mobile Electrons” • Metallic Bond = attraction between positive metal kernels and electrons in electron sea. Electrons can be attracted to many cations, not just 1.
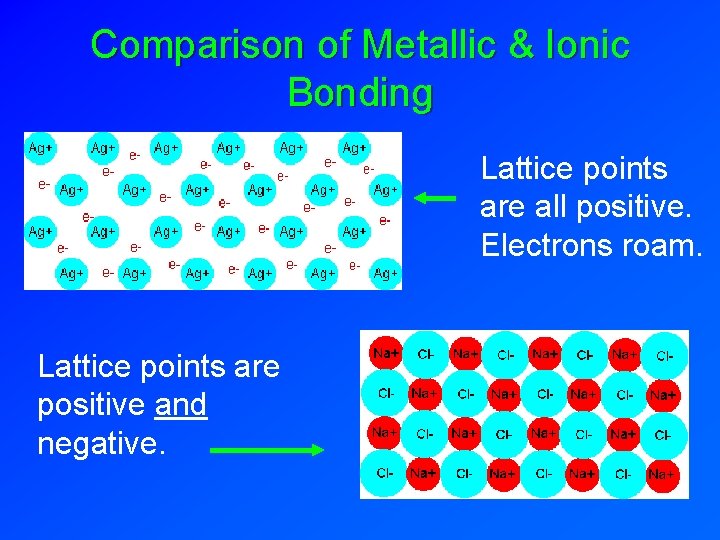
Comparison of Metallic & Ionic Bonding Lattice points are all positive. Electrons roam. Lattice points are positive and negative.
Properties of metals • Electrons can move so they conduct a current. • Electron sea holds the metal together when it’s deformed.

Metallic Bonds • Pretty strong. All metals but Hg are solids at room temperature. • Most have moderately high melting points & high boiling points.

Deformation of Metals
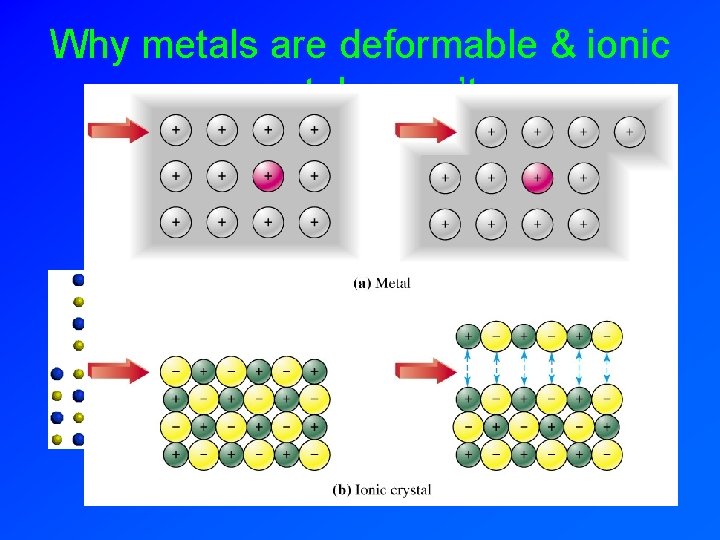
Why metals are deformable & ionic crystals aren’t:

Deformation of Metals • Applied force moves metal cations. • Cations are held together by electron sea.

Alloys • Mixture of elements that has metallic properties. • Adjust the mixture to get desired properties. • Can be substitutional or interstitial

- Slides: 16