Metallic Bonding Metallic Bond n n n Occurs

Metallic Bonding
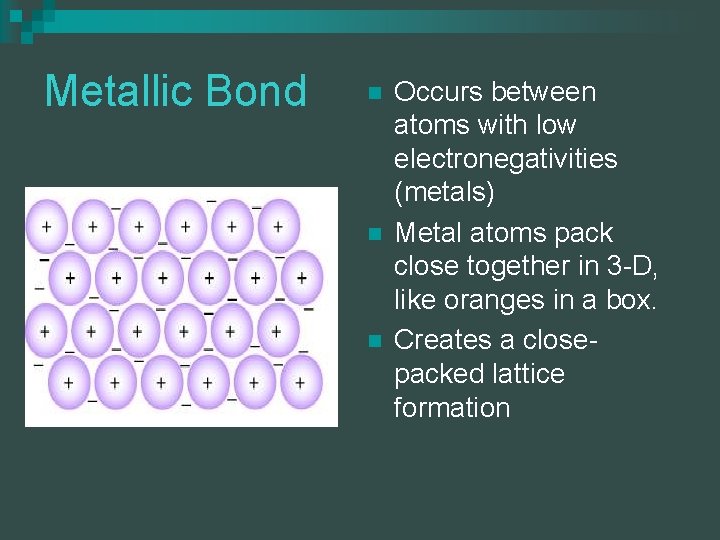
Metallic Bond n n n Occurs between atoms with low electronegativities (metals) Metal atoms pack close together in 3 -D, like oranges in a box. Creates a closepacked lattice formation

n n n Many metals have an unfilled outer orbital In an effort to be stable, their outer electrons become delocalized amongst all atoms No valence electrons belong to one atom, all are shared The electrons move around throughout the piece of metal. Metallic bonds are not ions, but nuclei with moving electrons http: //cd 1. edb. hkedcity. net/cd/science/che mistry/resource/animations/metallic_bond/ metallic. html This is called a “sea of electrons”
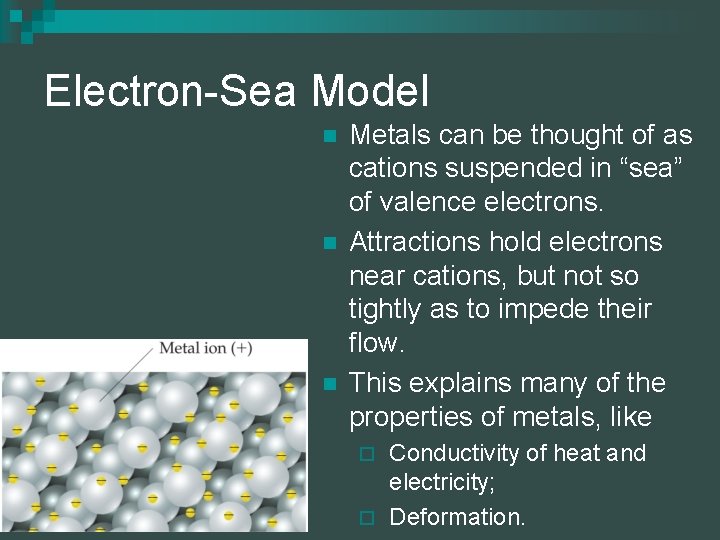
Electron-Sea Model n n n Metals can be thought of as cations suspended in “sea” of valence electrons. Attractions hold electrons near cations, but not so tightly as to impede their flow. This explains many of the properties of metals, like Conductivity of heat and electricity; ¨ Deformation. ¨ © 2009, Prentice-Hall, Inc.
Physical Properties Conductivity n Delocalized electrons are free to move so when a potential difference is applied they can carry the current (electrons) along n Mobile electrons also mean they can transfer heat well Ductility n Delocalized electrons allow metals to be stretched into a wire Luster n The interaction between the mobile electrons with light makes them shiny (luster)

Malleability n n n The electrons are attracted to the nuclei and are moving around constantly. The layers of the metal atoms can easily slide past each other without the need to break the bonds in the metal Gold is extremely malleable that 1 gram can be hammered into a sheet that is only 230 atoms thick (70 nm)
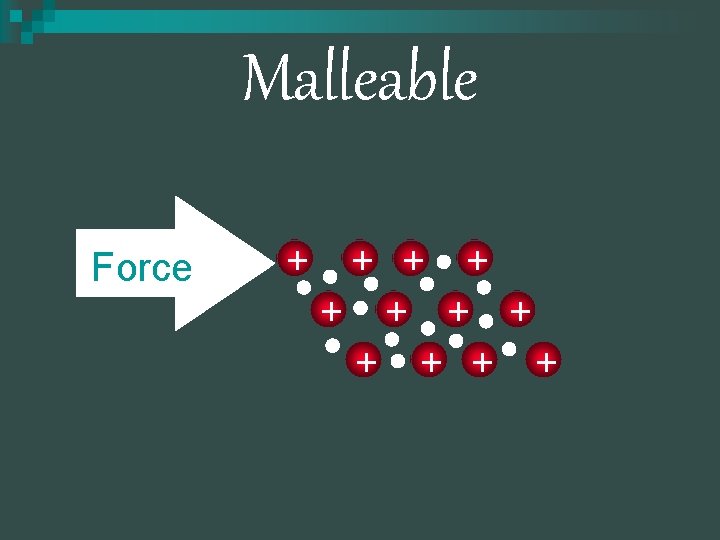
Malleable Force + + +
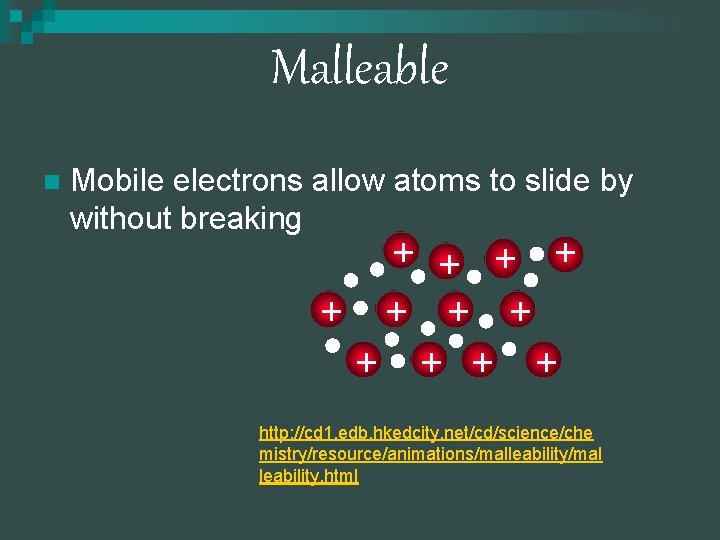
Malleable n Mobile electrons allow atoms to slide by without breaking + + + http: //cd 1. edb. hkedcity. net/cd/science/che mistry/resource/animations/malleability/mal leability. html

Melting/Boiling points n n n Metals melt at a moderately high temperatures Metals boil at an extremely high temperature because it requires the cations and its electrons to break away from the others, which takes a ton of energy. Gallium can melt in your hand at 29. 8 o. C, but it boils at 2400 o. C!

Alloys n n Alloy = mixture of elements with metallic properties Mixing one metal with other metal(s) or non metal(s) often enhances its properties ¨ Steel is stronger than pure iron because the carbon prevents the delocalized electrons to move so readily. ¨ If too much carbon is added then the metal is brittle. n n Alloys are generally less malleable and ductile than the original metal(s) Some alloys are made by melting and mixing two or more metals ¨ Bronze = copper and zinc ¨ Stainless Steel = iron, carbon, ¨ Carbide = Tungsten + Carbon and nickel
- Slides: 10