Metallic Bonding A metallic bond is the attraction
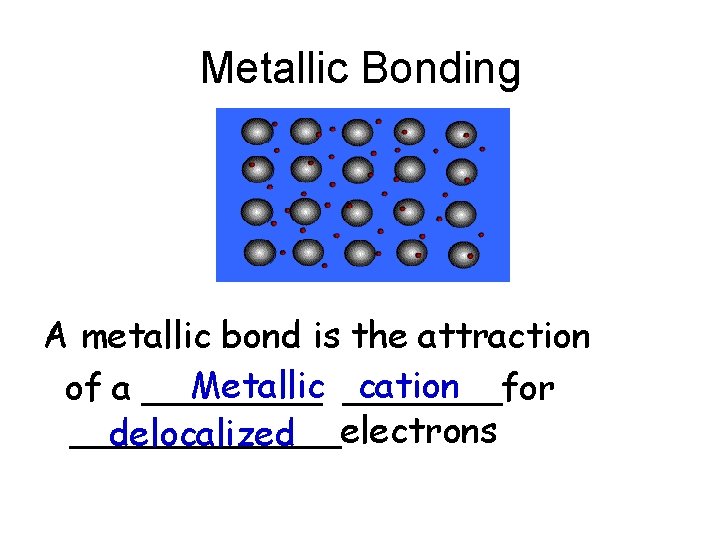
Metallic Bonding A metallic bond is the attraction Metallic _______for cation of a ____________electrons delocalized

Electron Sea Model proposes that the nuclei of the metallic cations hold onto their correct ____ of _____, but they may be any valence e- of a neighboring nucleus. They are NOT ______ or _____; they are floating in a sea above the _______ and can move freely from one atom to the next.

Electron Sea Model proposes that the nuclei of the metallic cations hold onto their correct number of electrons, but they may be any valence e- of a neighboring nucleus. They are NOT _______ or _____; they are floating in a sea above the ______and can move freely from one atom to the next.

Electron Sea Model proposes that the nuclei of the metallic cations hold onto their correct number of electrons, but they may be any valence e- of a neighboring nucleus. They are NOT shared or lost; they are floating in a sea above the nuclei and can move freely from one atom to the next.

delocalized electron = The valence e- of a metal that is free to move easily from one cation to another Attractions between positive cations and the negative “sea of e-“ hold the metal atoms together in a lattice.
Properties of metals • • • med. high melting points high boiling points malleable and ductile heat conductors electrical conductors
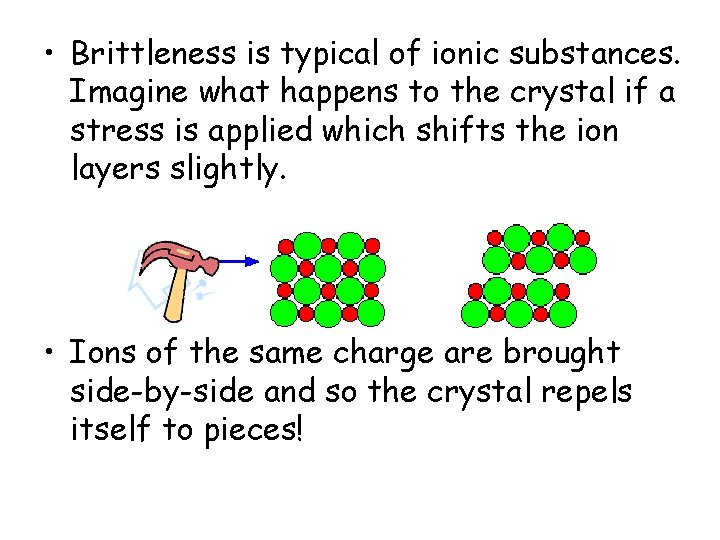
• Brittleness is typical of ionic substances. Imagine what happens to the crystal if a stress is applied which shifts the ion layers slightly. • Ions of the same charge are brought side-by-side and so the crystal repels itself to pieces!
Malleability of metals Whereas ionic cmpds shatter when hit with a mallet (hammer), metallic cmpds can be flattened and shaped. Why?

As the number of delocalized electrons increases, hardness & strength increase. In the transition metals, mobile electrons consist of both the valence electrons (highest s) and the inner d electrons.
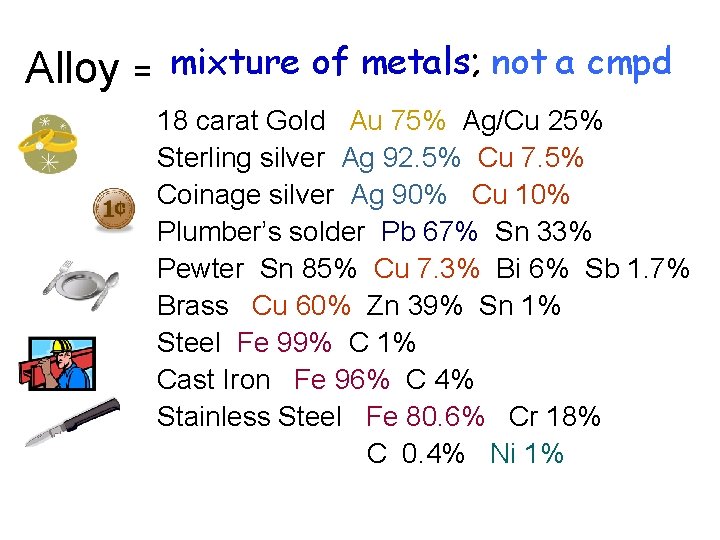
Alloy = mixture of metals; not a cmpd 18 carat Gold Au 75% Ag/Cu 25% Sterling silver Ag 92. 5% Cu 7. 5% Coinage silver Ag 90% Cu 10% Plumber’s solder Pb 67% Sn 33% Pewter Sn 85% Cu 7. 3% Bi 6% Sb 1. 7% Brass Cu 60% Zn 39% Sn 1% Steel Fe 99% C 1% Cast Iron Fe 96% C 4% Stainless Steel Fe 80. 6% Cr 18% C 0. 4% Ni 1%
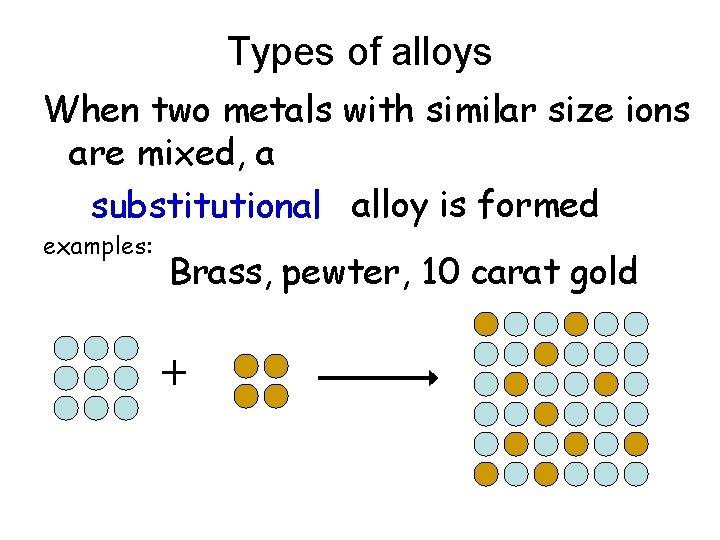
Types of alloys When two metals with similar size ions are mixed, a substitutional alloy is formed examples: Brass, pewter, 10 carat gold
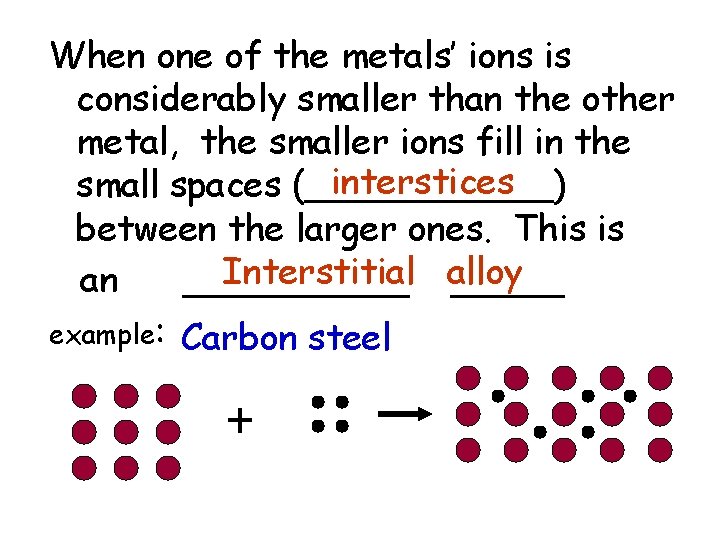
When one of the metals’ ions is considerably smaller than the other metal, the smaller ions fill in the interstices small spaces (______) between the larger ones. This is Interstitial alloy an _____ example: Carbon steel

Summary • “Sea of electrons” are reason for a metal’s properties • Alloys are mixtures of metals • Substitutional alloys have similar sized elements • Interstitial alloys have different sized elements • Metals are malleable (can be flattened) and ductile (can be drawn into wires), and have luster (are shiny)
- Slides: 13