Metal Reactivities Lab Introduction chchange investigate the relative
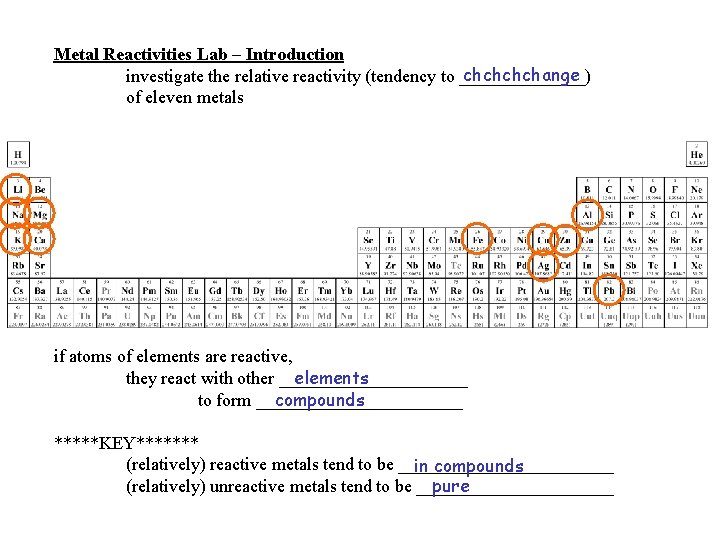
Metal Reactivities Lab – Introduction chchange investigate the relative reactivity (tendency to _______) of eleven metals if atoms of elements are reactive, elements they react with other ___________ to form ____________ compounds *****KEY******* (relatively) reactive metals tend to be ____________ in compounds pure (relatively) unreactive metals tend to be ___________

metal atoms lose OR gain electrons when they react with nonmetals (the nonmetal atoms lose OR gain electrons) **KEY** positive ion (cation) so, … in compounds, the metal is found as a _____________ negative ion (anion) the nonmetal is found as a ___________ example: pure sodium – Na and pure chlorine – Cl react to form the video link compound sodium chloride e. Na Cl Na+ and Cl- (or Na. Cl)
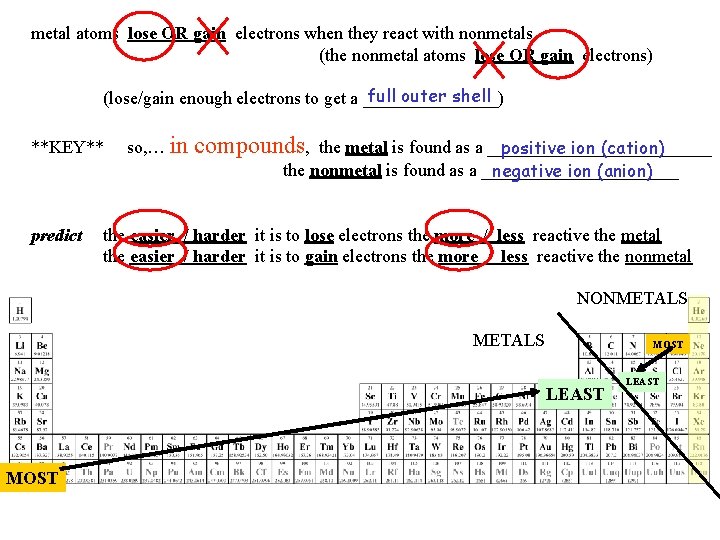
metal atoms lose OR gain electrons when they react with nonmetals (the nonmetal atoms lose OR gain electrons) full outer shell (lose/gain enough electrons to get a ________) **KEY** predict so, … in compounds, the metal is found as a _____________ positive ion (cation) the nonmetal is found as a ___________ negative ion (anion) the easier / harder it is to lose electrons the more / less reactive the metal the easier / harder it is to gain electrons the more / less reactive the nonmetal NONMETALS MOST LEAST
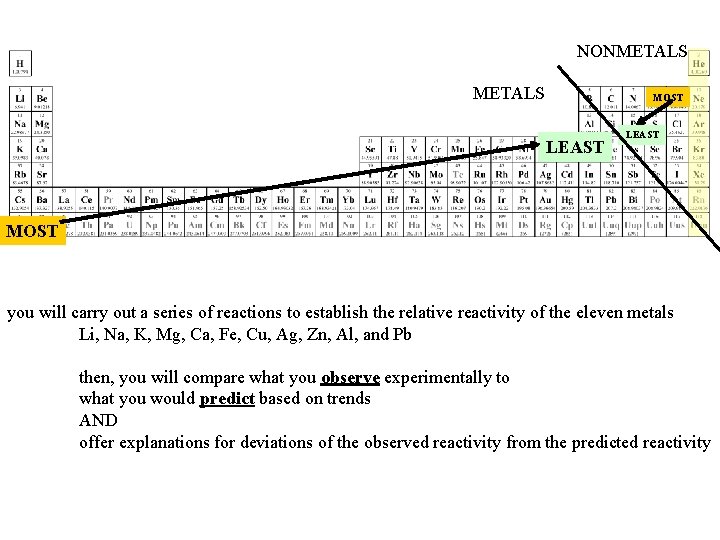
NONMETALS MOST LEAST MOST you will carry out a series of reactions to establish the relative reactivity of the eleven metals Li, Na, K, Mg, Ca, Fe, Cu, Ag, Zn, Al, and Pb then, you will compare what you observe experimentally to what you would predict based on trends AND offer explanations for deviations of the observed reactivity from the predicted reactivity
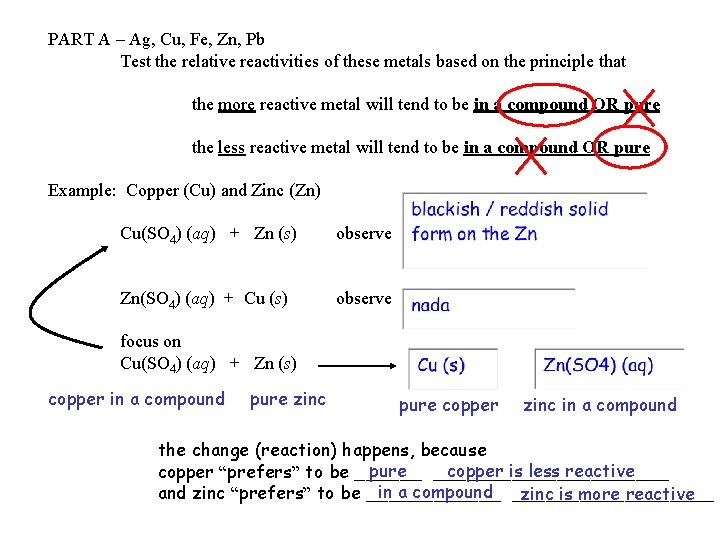
PART A – Ag, Cu, Fe, Zn, Pb Test the relative reactivities of these metals based on the principle that the more reactive metal will tend to be in a compound OR pure the less reactive metal will tend to be in a compound OR pure Example: Copper (Cu) and Zinc (Zn) Cu(SO 4) (aq) + Zn (s) observe Zn(SO 4) (aq) + Cu (s) observe focus on Cu(SO 4) (aq) + Zn (s) copper in a compound pure zinc pure copper zinc in a compound the change (reaction) happens, because pure ___________ copper is less reactive copper “prefers” to be ______ in a compound _________ and zinc “prefers” to be ______ zinc is more reactive
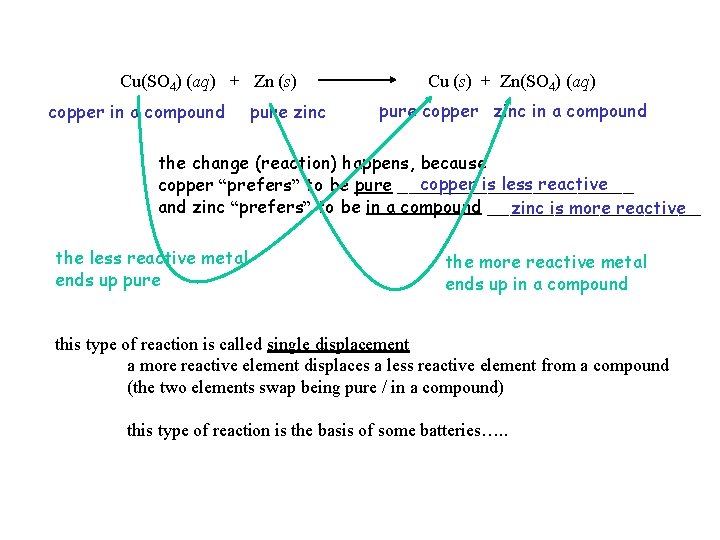
Cu(SO 4) (aq) + Zn (s) copper in a compound pure zinc Cu (s) + Zn(SO 4) (aq) pure copper zinc in a compound the change (reaction) happens, because copper is less reactive copper “prefers” to be pure ___________ and zinc “prefers” to be in a compound __________ zinc is more reactive the less reactive metal ends up pure the more reactive metal ends up in a compound this type of reaction is called single displacement a more reactive element displaces a less reactive element from a compound (the two elements swap being pure / in a compound) this type of reaction is the basis of some batteries…. .
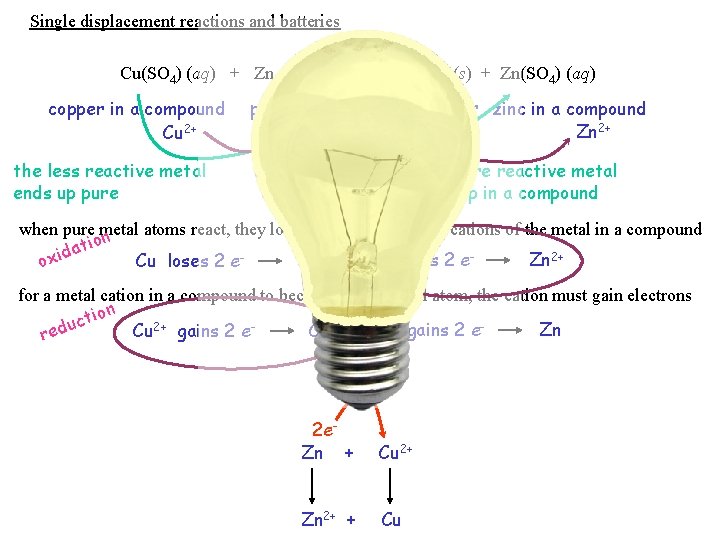
Single displacement reactions and batteries Cu(SO 4) (aq) + Zn (s) copper in a compound Cu 2+ Cu (s) + Zn(SO 4) (aq) pure zinc pure copper zinc in a compound Zn 2+ the less reactive metal ends up pure the more reactive metal ends up in a compound when pure metal atoms react, they lose electrons cations of the metal in a compound n o ati d i Zn loses 2 e. Zn 2+ Cu loses 2 e. Cu 2+ ox for a metal cation in a compound to become a pure metal atom, the cation must gain electrons tion c u Zn 2+ gains 2 e. Zn Cu 2+ gains 2 e. Cu red 2 e. Zn + Cu 2+ Zn 2+ + Cu
Metal Reactivities Lab – Introduction chchange investigate the relative reactivity (tendency to _______) of eleven metals w e evi R if atoms of elements are reactive, elements they react with other ___________ to form ____________ compounds *****KEY******* (relatively) reactive metals tend to be ____________ in compounds pure (relatively) unreactive metals tend to be ___________
metal atoms lose OR gain electrons when they react with nonmetals (the nonmetal atoms lose OR gain electrons) full outer shell (lose/gain enough electrons to get a ________) **KEY** predict so, … in compounds, the metal is found as a _____________ positive ion (cation) the nonmetal is found as a ___________ negative ion (anion) the easier / harder it is to lose electrons the more / less reactive the metal the easier / harder it is to gain electrons the more / less reactive the nonmetal v Re NONMETALS iew METALS MOST LEAST
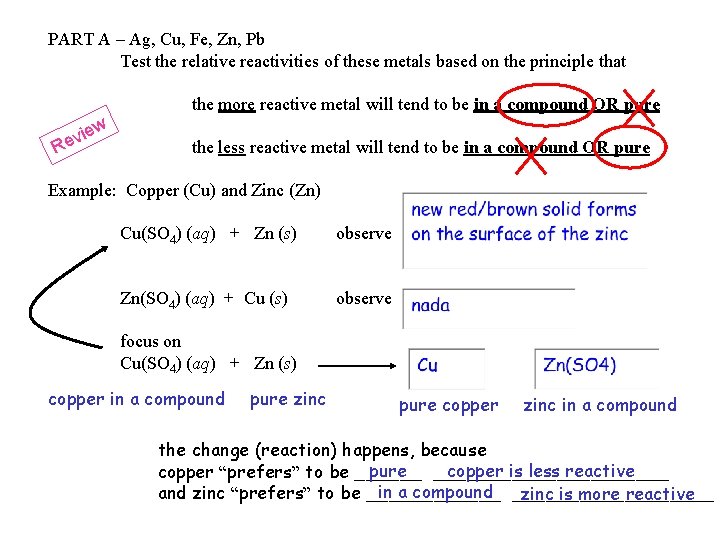
PART A – Ag, Cu, Fe, Zn, Pb Test the relative reactivities of these metals based on the principle that the more reactive metal will tend to be in a compound OR pure ew vi e R the less reactive metal will tend to be in a compound OR pure Example: Copper (Cu) and Zinc (Zn) Cu(SO 4) (aq) + Zn (s) observe Zn(SO 4) (aq) + Cu (s) observe focus on Cu(SO 4) (aq) + Zn (s) copper in a compound pure zinc pure copper zinc in a compound the change (reaction) happens, because pure ___________ copper is less reactive copper “prefers” to be ______ in a compound _________ and zinc “prefers” to be ______ zinc is more reactive

Phew… right…. . so, …. . for PART A principle – the more reactive metal “wants” to be in a compound the less reactive metal “wants” to be pure to test for the relative reactivity of two metals, - start with one pure and one in a compound - combine - look for evidence of the “swap” (single displacement) key evidence set up looks like….
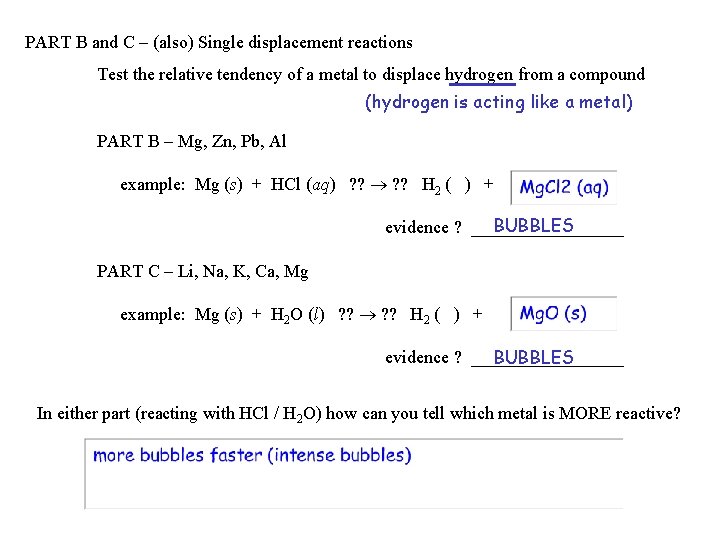
PART B and C – (also) Single displacement reactions Test the relative tendency of a metal to displace hydrogen from a compound (hydrogen is acting like a metal) PART B – Mg, Zn, Pb, Al example: Mg (s) + HCl (aq) ? ? H 2 ( ) + BUBBLES evidence ? _________ PART C – Li, Na, K, Ca, Mg example: Mg (s) + H 2 O (l) ? ? H 2 ( ) + evidence ? _________ BUBBLES In either part (reacting with HCl / H 2 O) how can you tell which metal is MORE reactive?
- Slides: 12