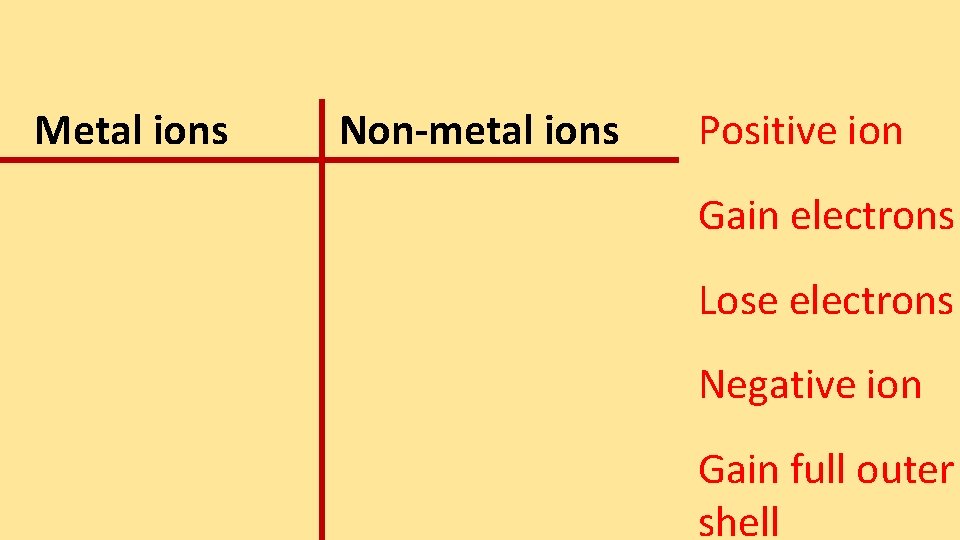
Metal ions Nonmetal ions Positive ion Gain electrons


- Slides: 16

Metal ions Non-metal ions Positive ion Gain electrons Lose electrons Negative ion Gain full outer shell

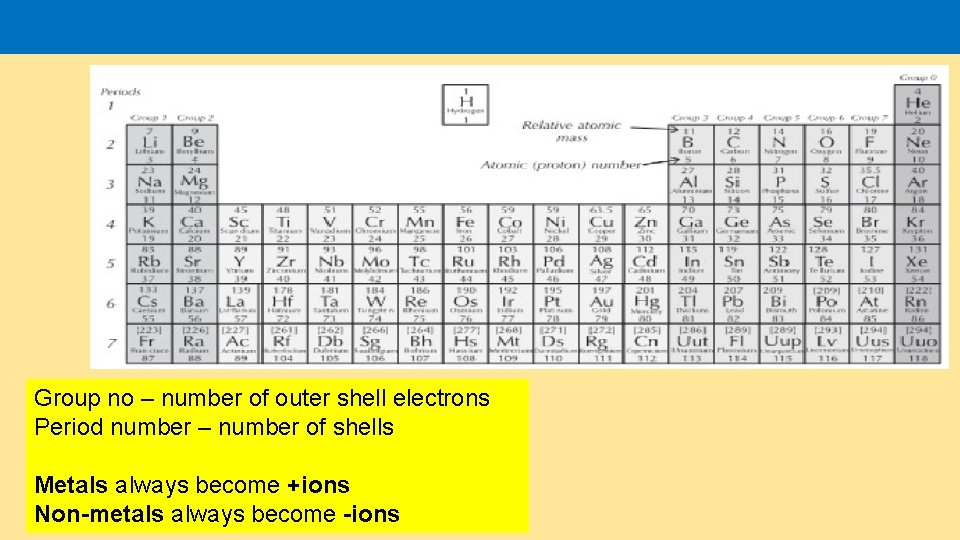
Group no – number of outer shell electrons Period number – number of shells Metals always become +ions Non-metals always become -ions

An atom that loses one or more electrons forms a positive ion. Metal atoms, such as sodium, magnesium and iron, form positive ions. lithium atom 2. 1 magnesium atom 2. 8. 2 aluminium atom 2. 8. 3 lithium ion [ 2 ] magnesium ion [ 2. 8 ] aluminium ion [ 2. 8 ] = Li+ = Mg 2+ = Al 3+

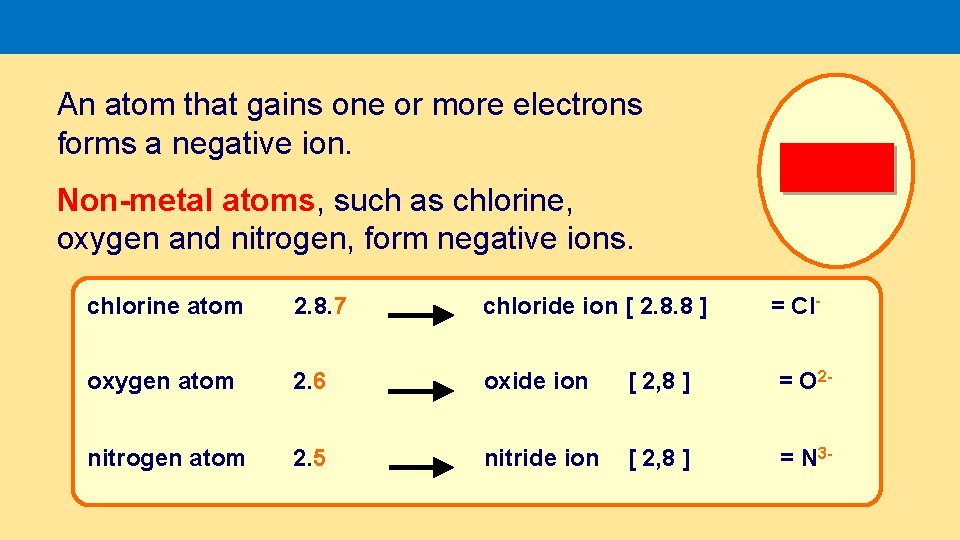
An atom that gains one or more electrons forms a negative ion. Non-metal atoms, such as chlorine, oxygen and nitrogen, form negative ions. chlorine atom 2. 8. 7 chloride ion [ 2. 8. 8 ] = Cl- oxygen atom 2. 6 oxide ion [ 2, 8 ] = O 2 - nitrogen atom 2. 5 nitride ion [ 2, 8 ] = N 3 -


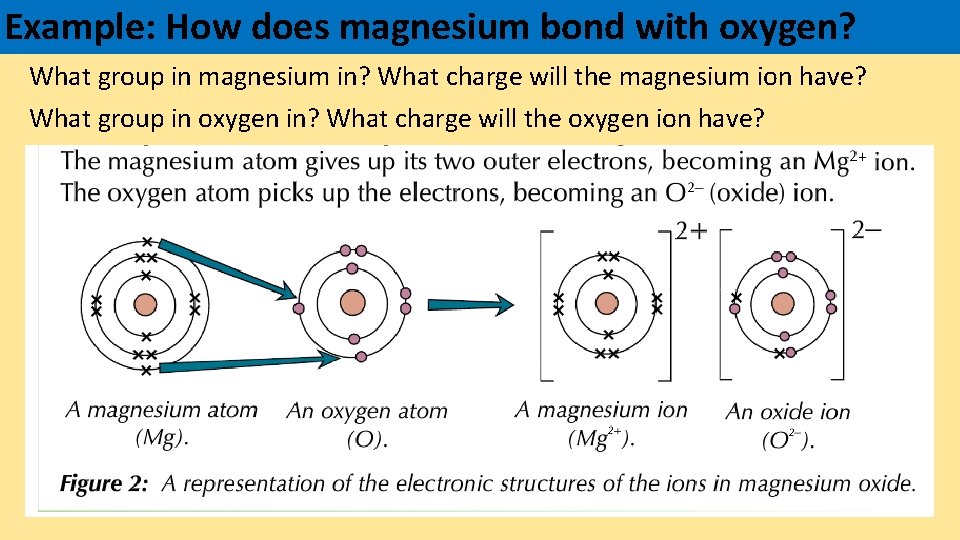
Example: How does magnesium bond with oxygen? What group in magnesium in? What charge will the magnesium ion have? What group in oxygen in? What charge will the oxygen ion have?

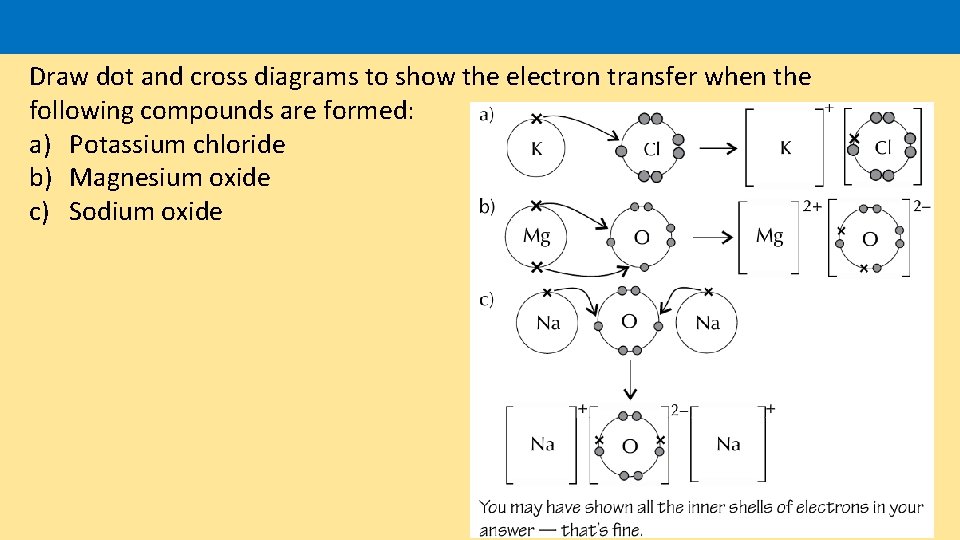
Draw dot and cross diagrams to show the electron transfer when the following compounds are formed: a) Potassium chloride b) Magnesium oxide c) Sodium oxide

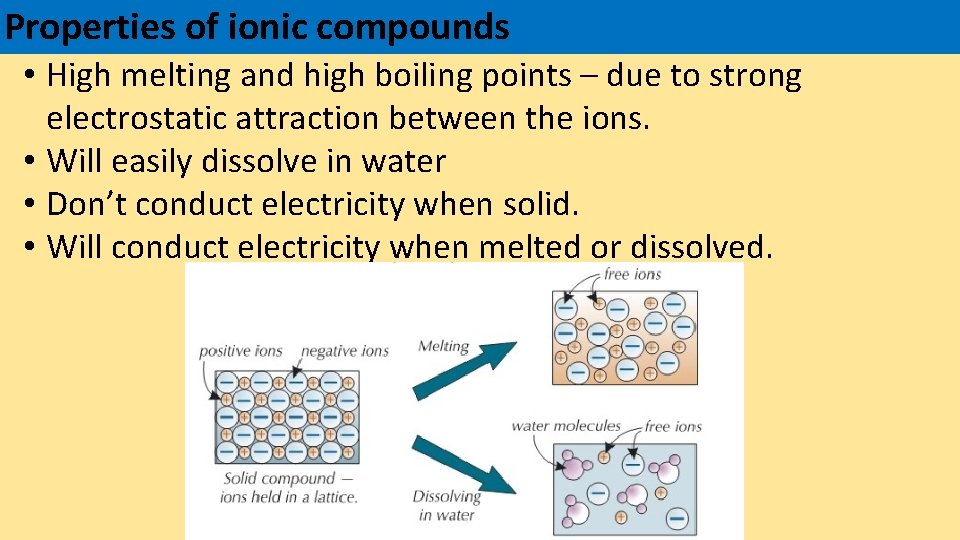
Properties of ionic compounds • High melting and high boiling points – due to strong electrostatic attraction between the ions. • Will easily dissolve in water • Don’t conduct electricity when solid. • Will conduct electricity when melted or dissolved.


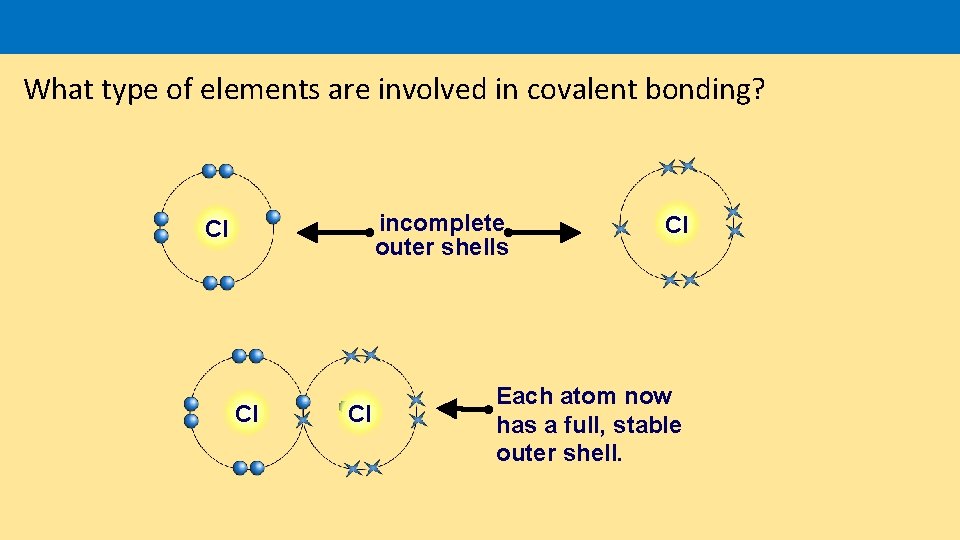
What type of elements are involved in covalent bonding? incomplete outer shells Cl Cl Each atom now has a full, stable outer shell.

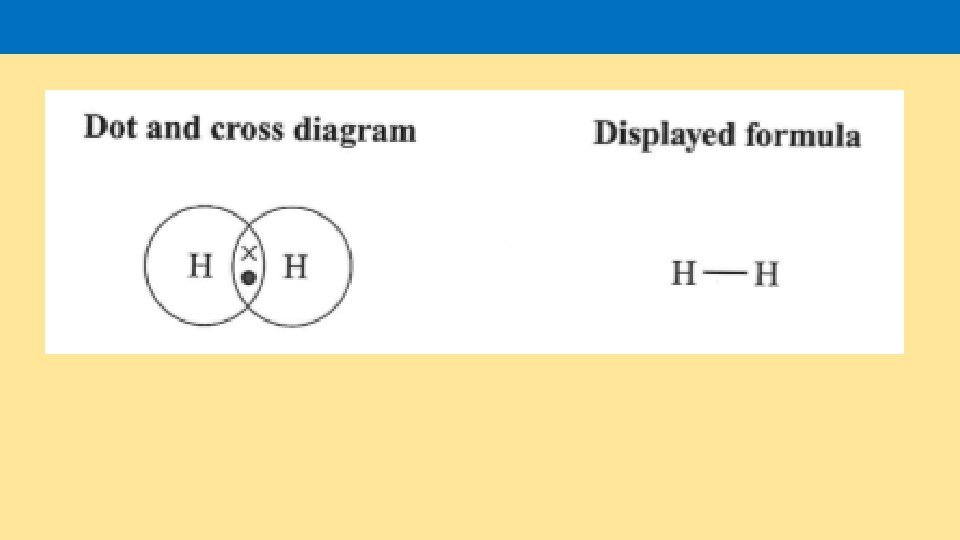
Draw a dot and cross diagram showing the bonding of two oxygen atoms. Draw a dot and cross diagram showing the bonding of one hydrogen atom and one chlorine atom. Draw a dot and cross diagram showing for methane CH 4. Draw a dot and cross diagram showing for carbon dioxide.


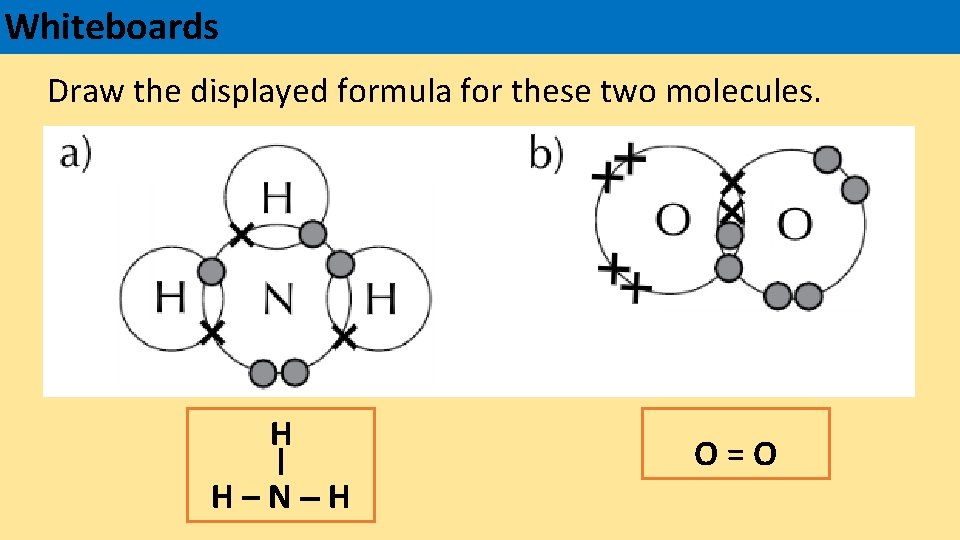
Whiteboards Draw the displayed formula for these two molecules. H H–N–H O=O

What are simple covalent structures Covalent molecules that contain only a few atoms are called simple covalent structures. Most substances that contain simple covalent molecules have low melting and boiling points and are therefore liquids or gases at room temperature, e. g. water, oxygen, carbon dioxide, chlorine and hydrogen. Why?

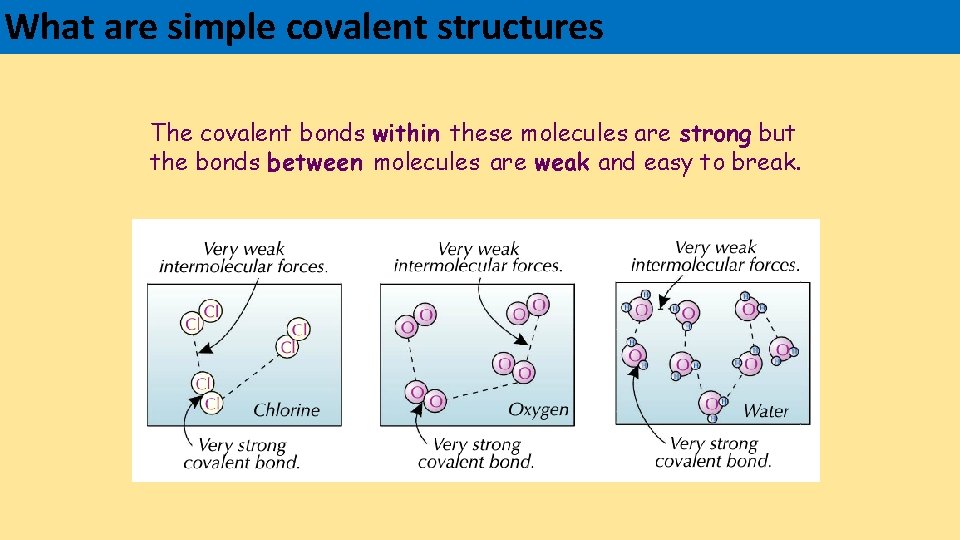
What are simple covalent structures The covalent bonds within these molecules are strong but the bonds between molecules are weak and easy to break.

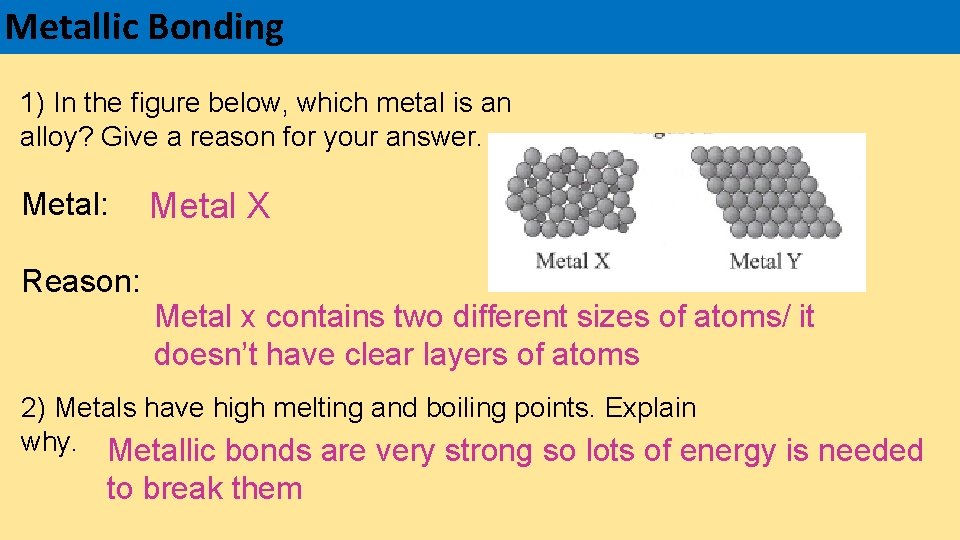
Metallic Bonding 1) In the figure below, which metal is an alloy? Give a reason for your answer. Metal: Reason: Metal X Metal x contains two different sizes of atoms/ it doesn’t have clear layers of atoms 2) Metals have high melting and boiling points. Explain why. Metallic bonds are very strong so lots of energy is needed to break them