Metabolism the chemical reactions of a cell 1
Metabolism: the chemical reactions of a cell 1 • All organisms need two things with which to grow: – Raw materials (especially carbon atoms) – Energy. • Types of metabolic reactions: – Anabolism: biosynthesis; reactions that create large/complex molecules from smaller, simpler ones. Use raw materials and energy. – Catabolism: degradation; reactions that break down large/complex molecules, used to generate energy for use and to produce smaller, building block molecules.

Energy: where from? What for? • Chemotrophs vs. phototrophs – Chemotrophs get energy from molecules • Chemolithotrophs get energy from oxidation of inorganic substances. • Chemoorganotrophs get energy from oxidation of organic compunds (like we do). – Phototrophs get energy from sunlight • Energy is needed to power the cell – Biosynthesis to respond to environment, to grow – Active transport, motility, etc. 2

• • • Bacteria obtain energy through 3 oxidation/reduction reactions Oxidation: molecule gives up electrons Reduction: molecule accepts electrons Oxidation/reduction (redox) reactions always occur in pairs; if electrons are removed, they must go somewhere! Biological redox reactions usually involve PAIRS of electrons. Biological redox reactions often involve entire hydrogen atoms, not just the electrons (so called dehydrogenation reactions).
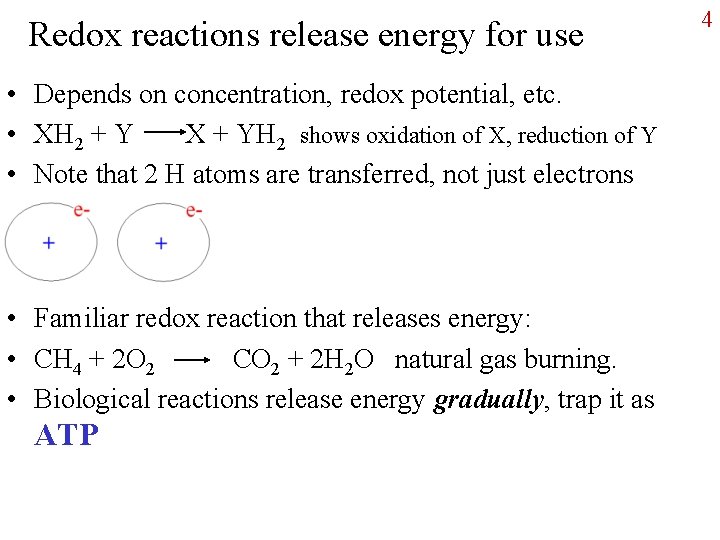
Redox reactions release energy for use • Depends on concentration, redox potential, etc. • XH 2 + Y X + YH 2 shows oxidation of X, reduction of Y • Note that 2 H atoms are transferred, not just electrons • Familiar redox reaction that releases energy: • CH 4 + 2 O 2 CO 2 + 2 H 2 O natural gas burning. • Biological reactions release energy gradually, trap it as ATP 4
Metabolic reactions require enzymes 5 • Reactions operate in pathways: A B C D Where A-D are different molecules Each step is catalyzed by a different enzyme. A Catalyst is something that speeds up a chemical reaction and is not consumed in the reaction, but can be re-used. Enzymes are biological catalysts; 99. 99% of them are proteins. Enzymes are very specific; a different one is required for each type of chemical reaction. Because the 3 -D shape of an enzyme is critical for its function, anything that alters that (heat, high salt, extreme p. H) will affect how fast or whether it works.
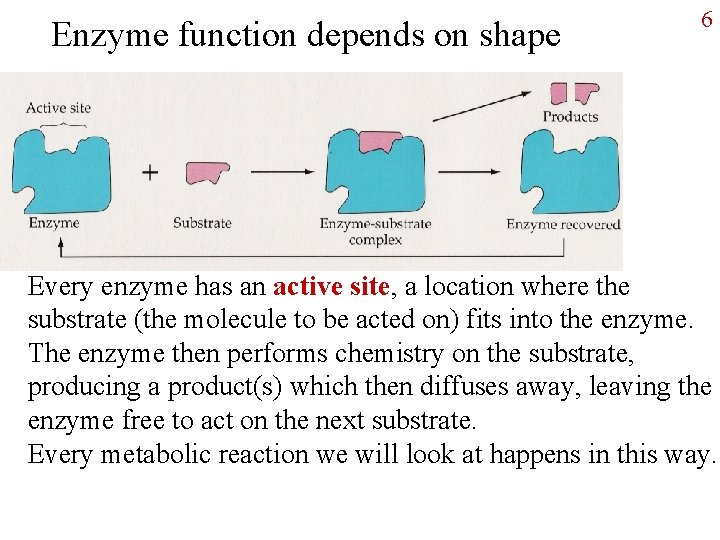
Enzyme function depends on shape 6 Every enzyme has an active site, a location where the substrate (the molecule to be acted on) fits into the enzyme. The enzyme then performs chemistry on the substrate, producing a product(s) which then diffuses away, leaving the enzyme free to act on the next substrate. Every metabolic reaction we will look at happens in this way.
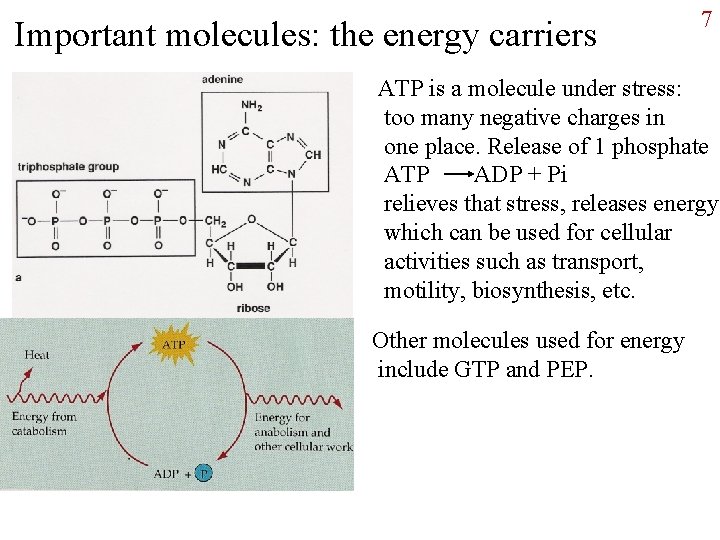
Important molecules: the energy carriers 7 ATP is a molecule under stress: too many negative charges in one place. Release of 1 phosphate ATP ADP + Pi relieves that stress, releases energy which can be used for cellular activities such as transport, motility, biosynthesis, etc. Other molecules used for energy include GTP and PEP.
Important molecules: the electron carriers 8 • The energy released in redox reactions is often thought of as the energy in the bonds between the H and the C; when a molecule is reduced by transfer of the H, the energy is conserved in that reduced molecule. • The most common electron carrier is NAD: – NAD + XH 2 X + NADH + H+ where NAD carries 2 e-, 1 H+ – Reduced NAD (NADH) is like poker chips, energy that can’t be spent, but can be “cashed in” later to make ATP (which can be “spent”, i. e. used as an energy source for cell activities). • Other electron carriers: NADP which is used to donate H for biosynthesis; also FAD.
More about Enzymes • Sometimes an enzyme needs help – Protein alone = apoenzyme – Helper molecule: cofactor • Could be inorganic like a metal ion (Fe+2) • Could be organic coenzyme (like Co. A, NAD) – Apoenzyme + cofactor = holoenzyme. – Cofactors have an effect on nutrition • Bacteria have certain mineral requirements. • Vitamins are cofactors that are needed in the “diet”. 9

Enzymes can be stopped • Conditions that disrupt the 3 D shape – Acidic, alkaline, high salt, high temperature, etc. – These conditions thus affect growth of cell also. • Inhibitory molecules affect enzymes – Competitive inhibitors • Fit in active site but are not changed; prevent normal substrate from binding, prevent reaction. – Non-competitive inhibitors • Bind permanently to active site or other site which changes molecular shape; prevents reaction. – Allosteric inhibitor: temporary binding, regulates. 10
- Slides: 10