Metabolism of Nonnutritional Substances n Biotransformation in liver
Metabolism of Non-nutritional Substances n Biotransformation in liver n Metabolism of bile acids n Biosynthesis of heme n Metabolism of bile pigments
SectionⅠ Biotransformation of liver n Some non-nutrient substances can be converted to more polar metabolites by various chemical reactions such as oxidation, reduction, hydrolysis, and conjugation, which are then excreted from the body. These processes mainly occur in liver.

n Sources of non-nutrients: * endogenous: hormones, neurotransmitters, amine, ammonia and bile pigment… * exogenous: drugs, food additives, toxicity and environmental pollutants, etc.
n Significance of biotransformation: 1. Increase solubility (polarity). Facilitate their excretion from the body. (excreted in the urine and bile) 2. Decrease or eliminate biological activity and toxicity 3. But some materials become less soluble and more toxic,increase their biological activity. (Dualism of detoxicification and toxicification) biotransformation Detoxification
n Major types of biotransformation reactions (1) Phase 1 reactions: oxidation reduction hydrolysis (2) Phase 2 reactions: conjugation
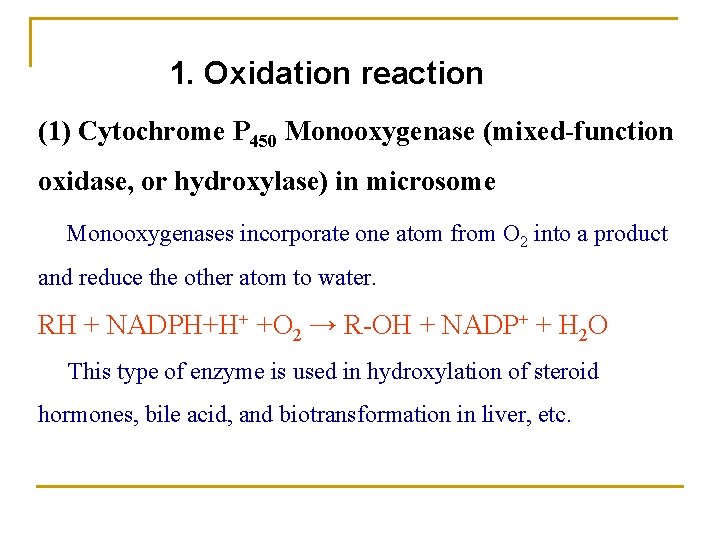
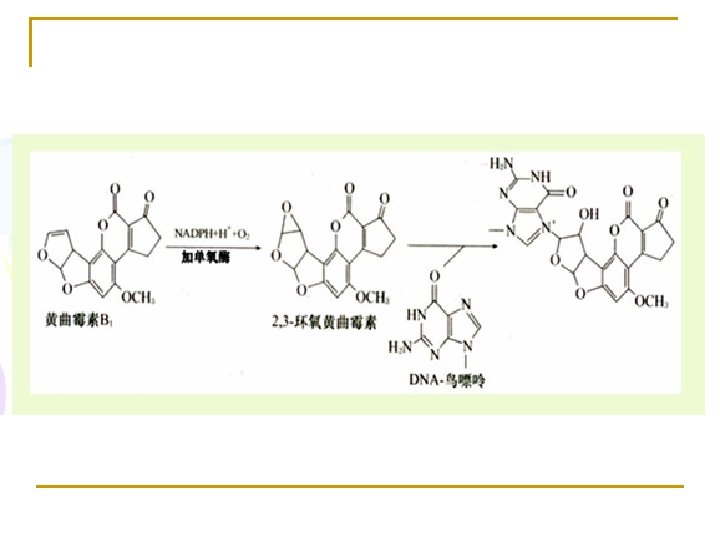
1. Oxidation reaction (1) Cytochrome P 450 Monooxygenase (mixed-function oxidase, or hydroxylase) in microsome Monooxygenases incorporate one atom from O 2 into a product and reduce the other atom to water. RH + NADPH+H+ +O 2 → R-OH + NADP+ + H 2 O This type of enzyme is used in hydroxylation of steroid hormones, bile acid, and biotransformation in liver, etc.
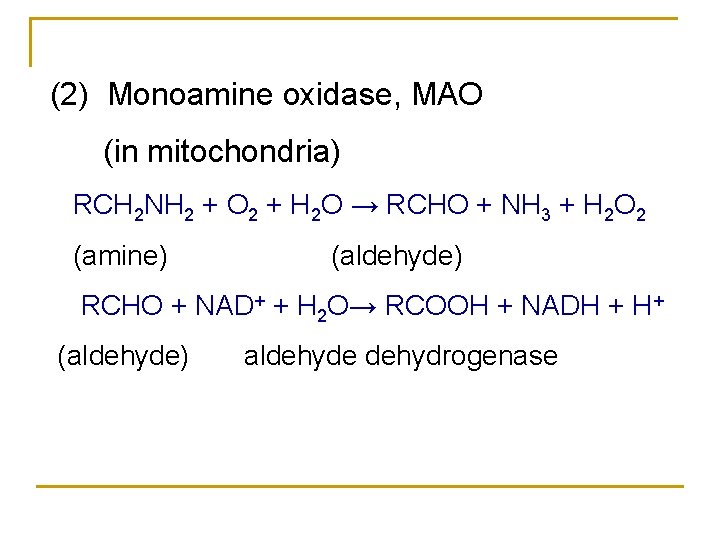
(2) Monoamine oxidase, MAO (in mitochondria) RCH 2 NH 2 + O 2 + H 2 O → RCHO + NH 3 + H 2 O 2 (amine) (aldehyde) RCHO + NAD+ + H 2 O→ RCOOH + NADH + H+ (aldehyde) aldehyde dehydrogenase
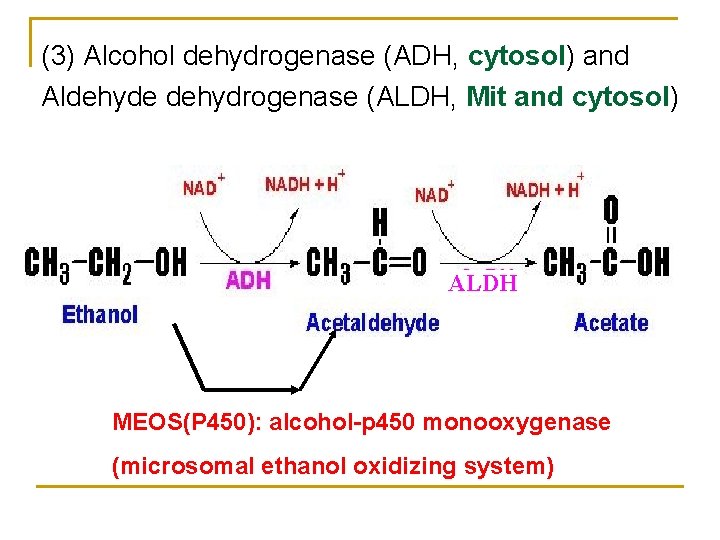
(3) Alcohol dehydrogenase (ADH, cytosol) and Aldehyde dehydrogenase (ALDH, Mit and cytosol) ALDH MEOS(P 450): alcohol-p 450 monooxygenase (microsomal ethanol oxidizing system)
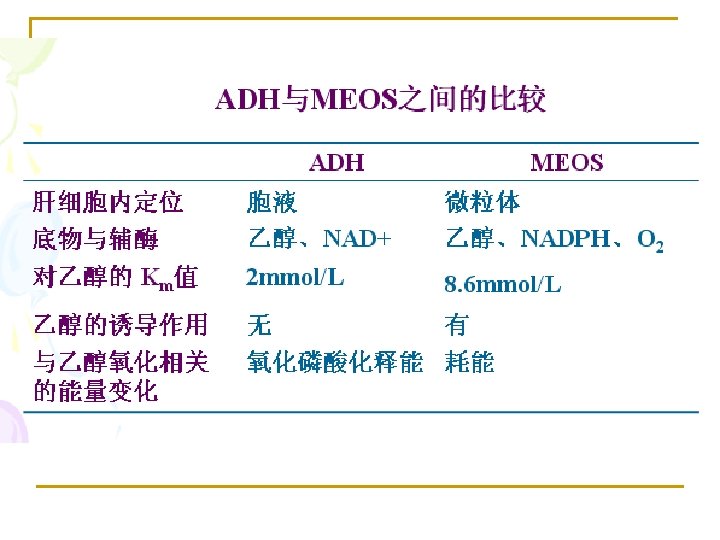
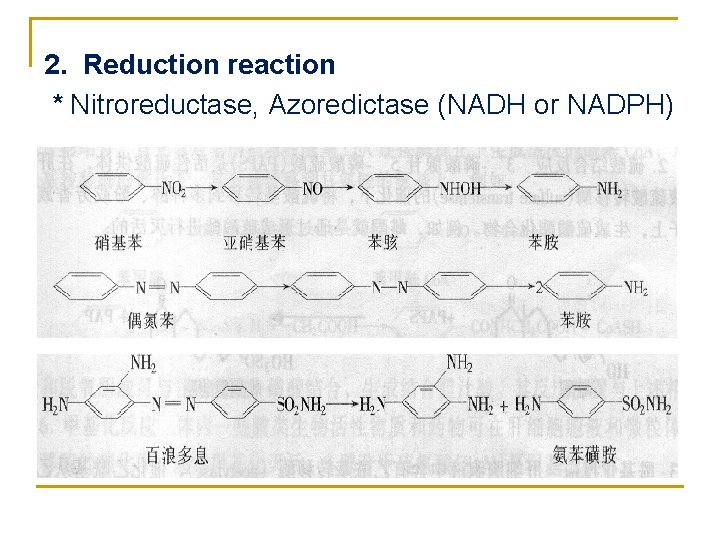
2. Reduction reaction * Nitroreductase, Azoredictase (NADH or NADPH)
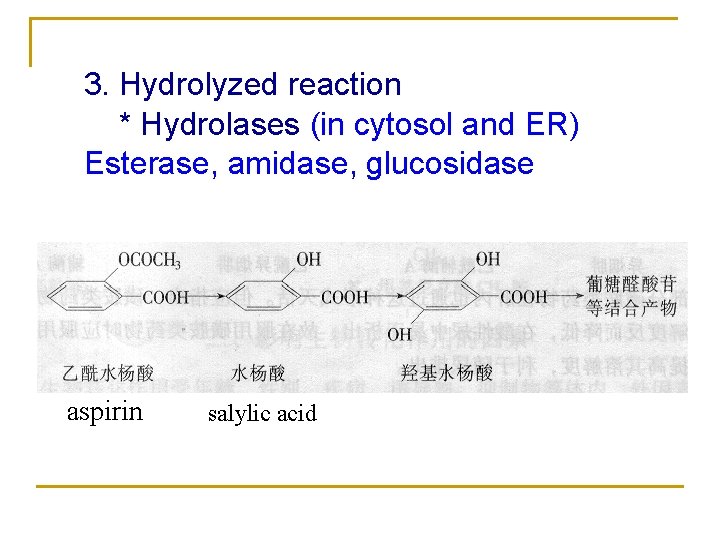
3. Hydrolyzed reaction * Hydrolases (in cytosol and ER) Esterase, amidase, glucosidase aspirin salylic acid
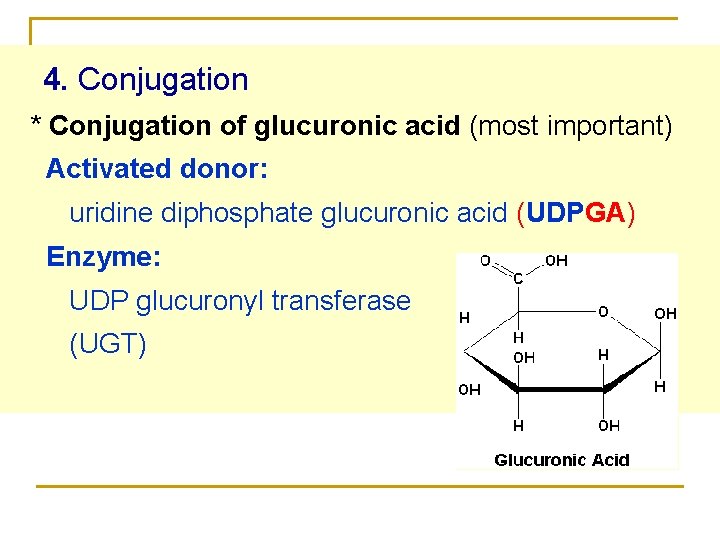
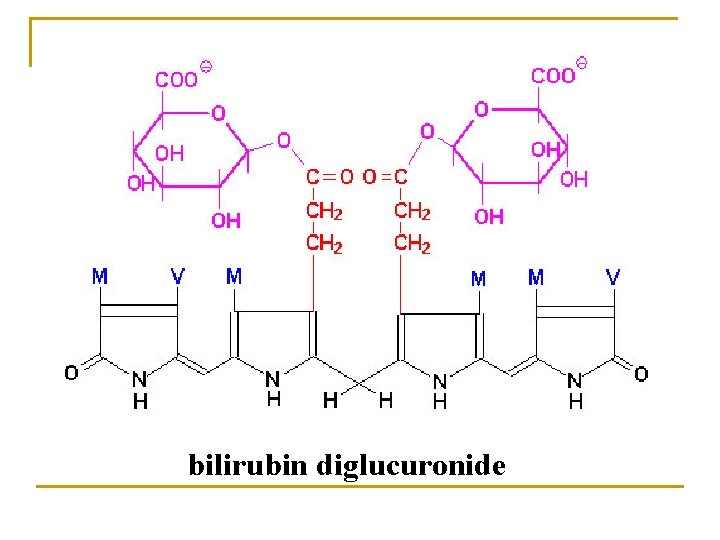
4. Conjugation * Conjugation of glucuronic acid (most important) Activated donor: uridine diphosphate glucuronic acid (UDPGA) Enzyme: UDP glucuronyl transferase (UGT)

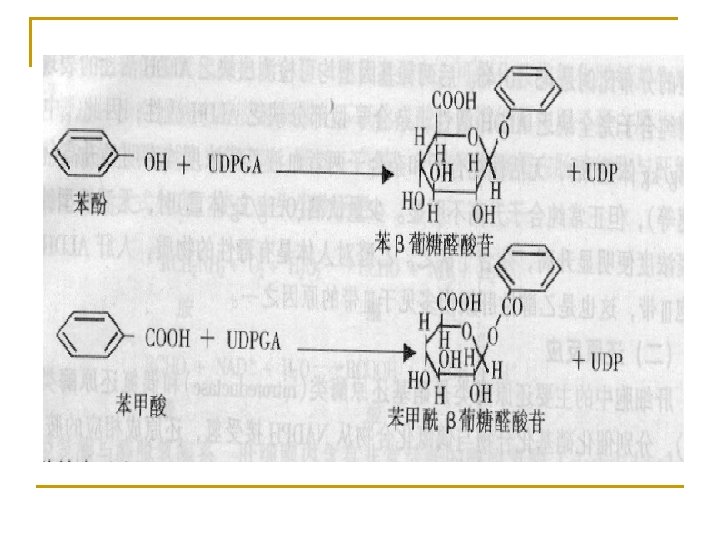
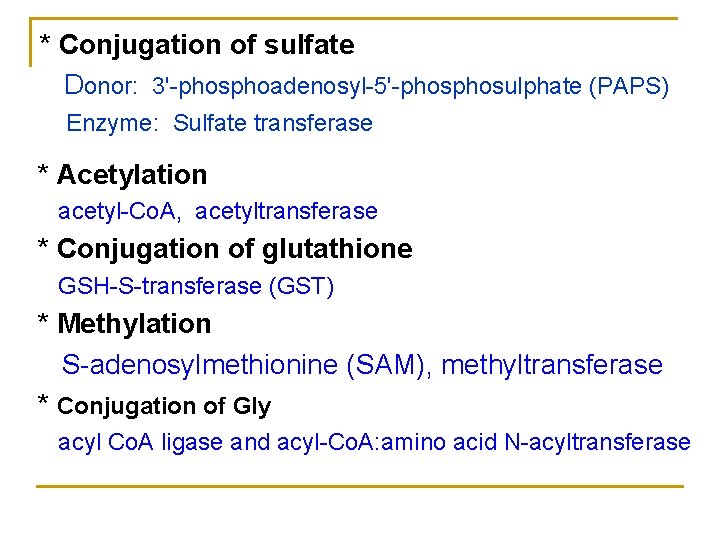
* Conjugation of sulfate Donor: 3'-phosphoadenosyl-5'-phosulphate (PAPS) Enzyme: Sulfate transferase * Acetylation acetyl-Co. A, acetyltransferase * Conjugation of glutathione GSH-S-transferase (GST) * Methylation S-adenosylmethionine (SAM), methyltransferase * Conjugation of Gly acyl Co. A ligase and acyl-Co. A: amino acid N-acyltransferase
n Factors that affect biotransformation (1) Age, sex, nutrition state, diseases and heredity (2) Inducers and inhibitors
Section Ⅱ Bile and Bile acid metabolism
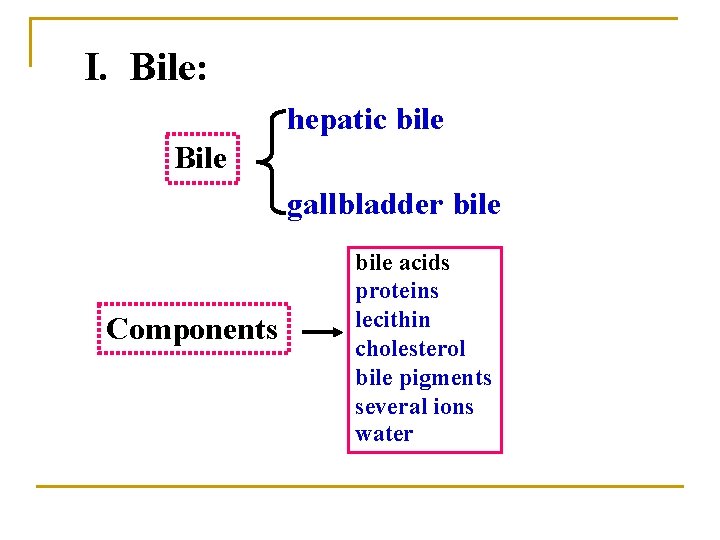
I. Bile: hepatic bile Bile gallbladder bile Components bile acids proteins lecithin cholesterol bile pigments several ions water
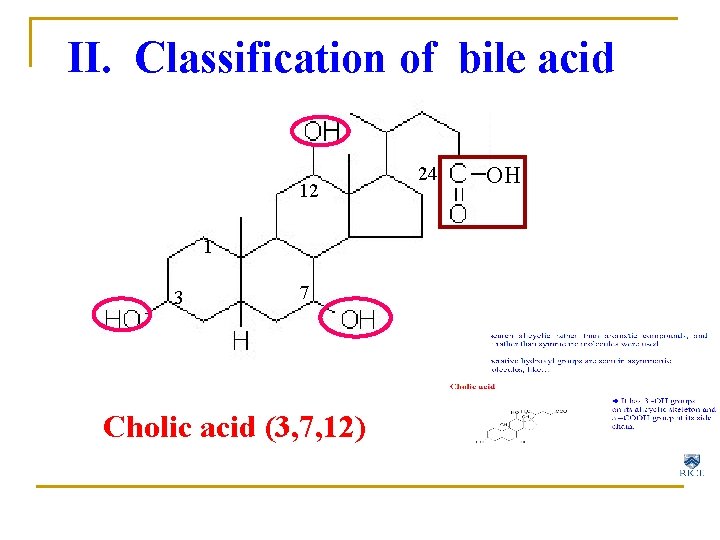
II. Classification of bile acid 12 1 3 7 Cholic acid (3, 7, 12) 24 OH
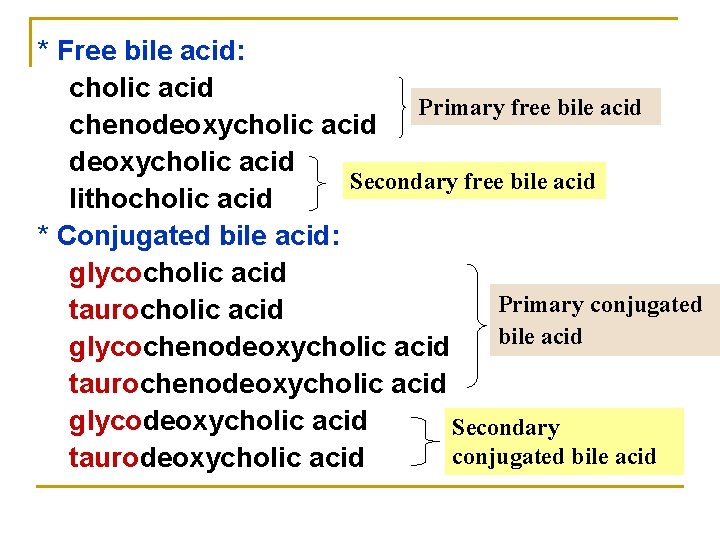
* Free bile acid: cholic acid Primary free bile acid chenodeoxycholic acid Secondary free bile acid lithocholic acid * Conjugated bile acid: glycocholic acid Primary conjugated taurocholic acid bile acid glycochenodeoxycholic acid taurochenodeoxycholic acid glycodeoxycholic acid Secondary conjugated bile acid taurodeoxycholic acid
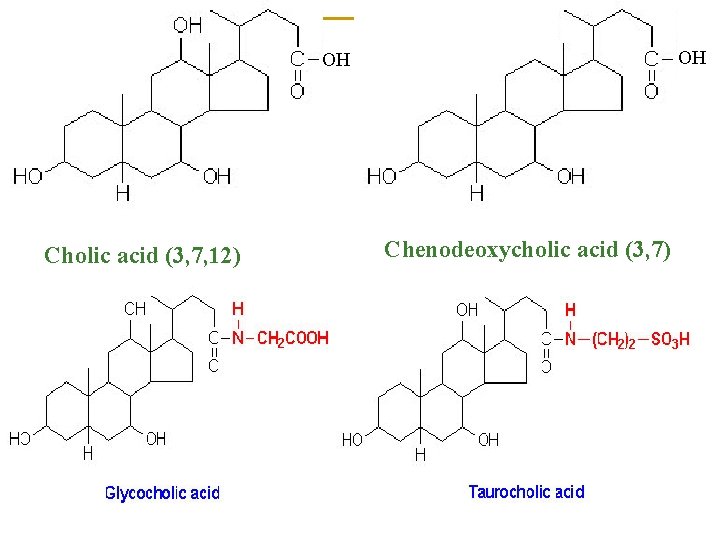
OH OH Cholic acid (3, 7, 12) Chenodeoxycholic acid (3, 7)
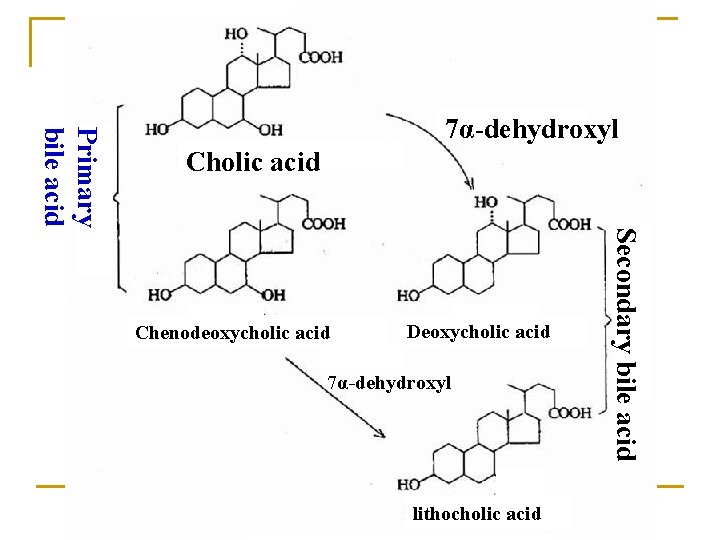
Primary bile acid 7α-dehydroxyl Cholic acid Deoxycholic acid 7α-dehydroxyl lithocholic acid Secondary bile acid Chenodeoxycholic acid

Ⅲ. Functions of bile acids • Facilitate digestion and absorption of dietary lipids and fat-soluble vitamins • Bile acids and phospholipids solubilize cholesterol in the bile, thereby preventing the precipitation of cholesterol in the gallbladder. (Bile salts + lecithin) ︰ cholesterol → 10 : 1
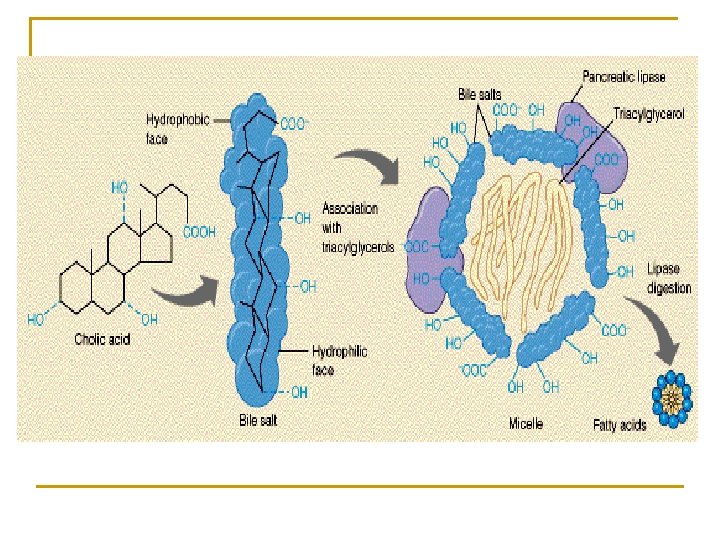

Ⅳ. Metabolism of bile acid and enterohepatic circulation of bile acid
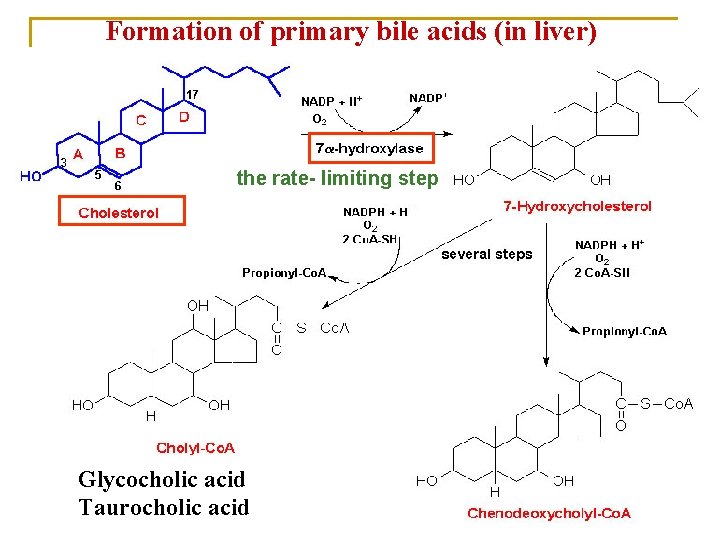
Formation of primary bile acids (in liver) the rate- limiting step Glycocholic acid Taurocholic acid
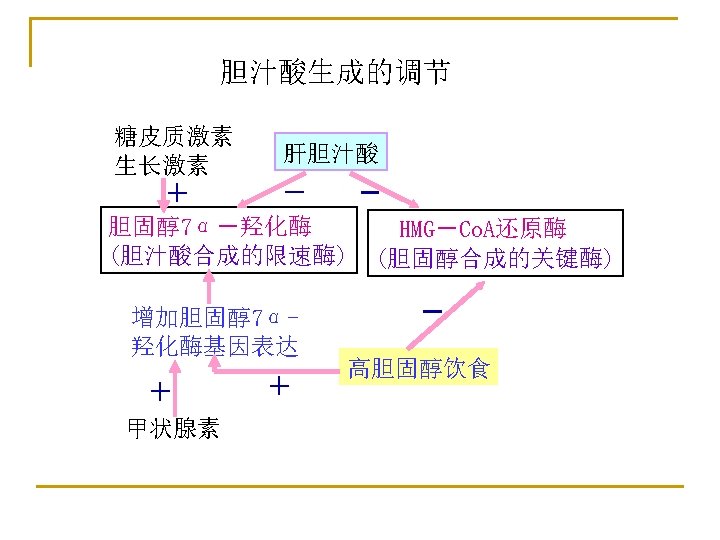
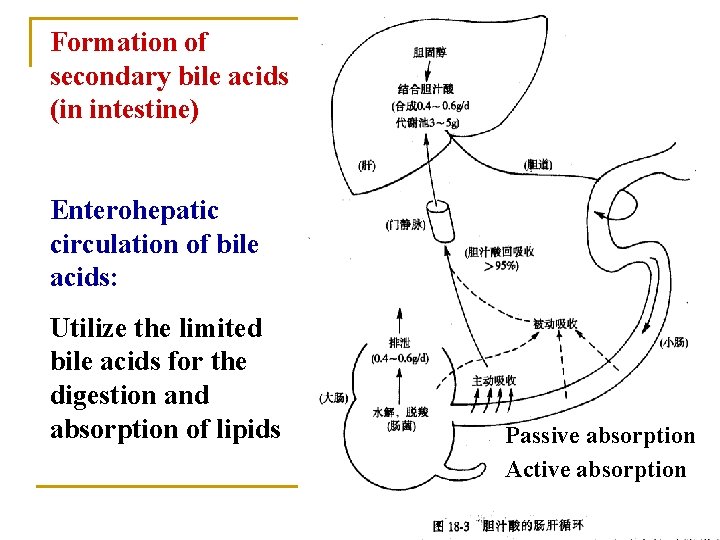
Formation of secondary bile acids (in intestine) Enterohepatic circulation of bile acids: Utilize the limited bile acids for the digestion and absorption of lipids Passive absorption Active absorption
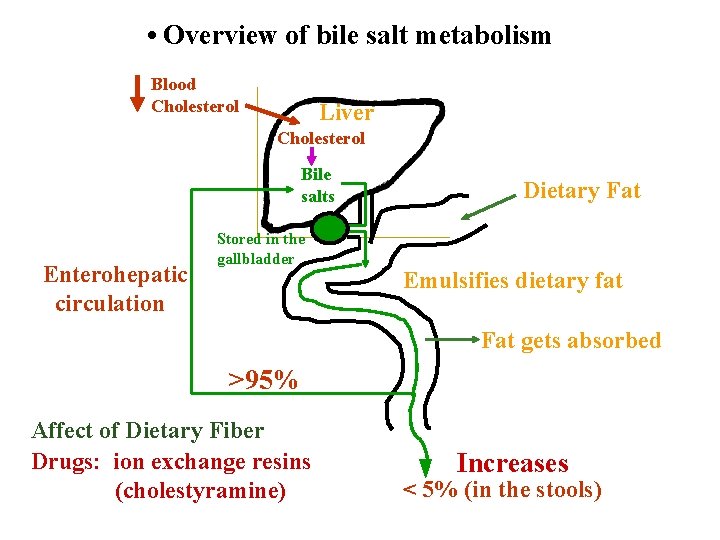
• Overview of bile salt metabolism Blood Cholesterol Liver Cholesterol Bile salts Enterohepatic circulation Stored in the gallbladder Dietary Fat Emulsifies dietary fat Fat gets absorbed >95% Affect of Dietary Fiber Drugs: ion exchange resins (cholestyramine) Increases < 5% (in the stools)
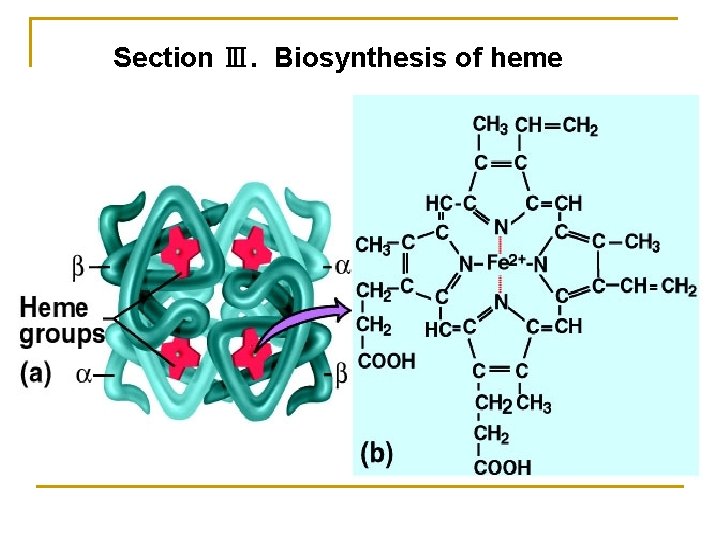
Section Ⅲ. Biosynthesis of heme
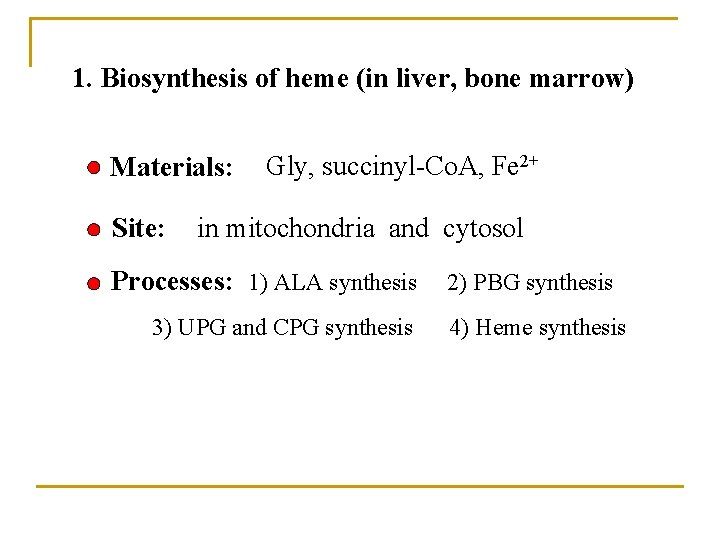
1. Biosynthesis of heme (in liver, bone marrow) Materials: Site: Gly, succinyl-Co. A, Fe 2+ in mitochondria and cytosol Processes: 1) ALA synthesis 3) UPG and CPG synthesis 2) PBG synthesis 4) Heme synthesis
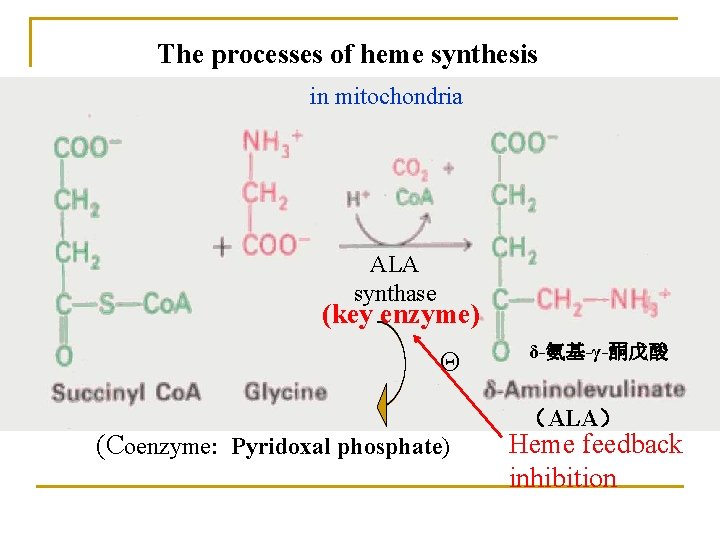
The processes of heme synthesis in mitochondria ALA synthase (key enzyme) Θ (Coenzyme: Pyridoxal phosphate) δ-氨基-γ-酮戊酸 (ALA) Heme feedback inhibition
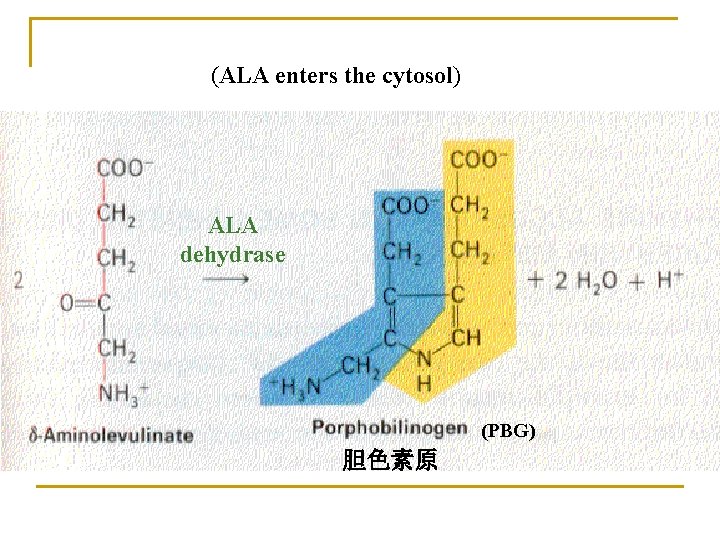
(ALA enters the cytosol) ALA dehydrase (PBG) 胆色素原
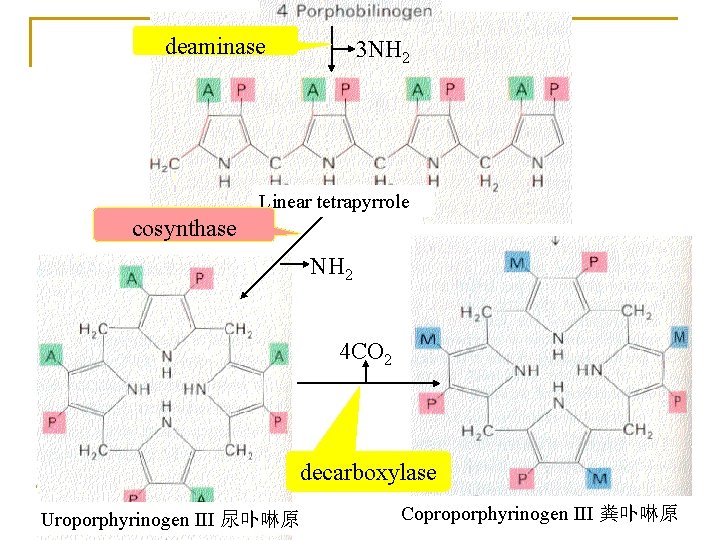
deaminase 3 NH 2 Linear tetrapyrrole cosynthase NH 2 4 CO 2 decarboxylase Uroporphyrinogen III 尿卟啉原 Coproporphyrinogen III 粪卟啉原
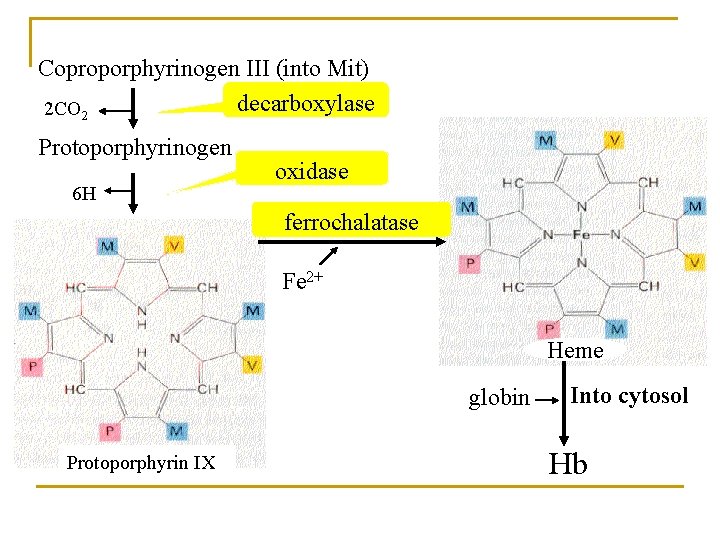
Coproporphyrinogen III (into Mit) decarboxylase 2 CO 2 Protoporphyrinogen 6 H oxidase ferrochalatase Fe 2+ Heme globin Protoporphyrin IX Into cytosol Hb
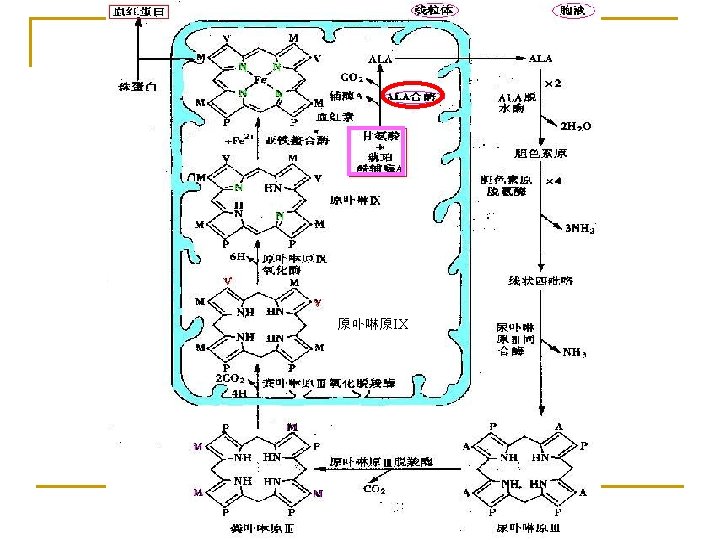
Summary ① Materials: succinyl-Co. A, glycine and Fe 2+. ② The initial reaction and the last three occur in mitochondria; The intermediate steps occur in the cytosol. ③ Synthesized mainly in liver and bone marrow but mature red blood cells lack mitochondria and are unable to synthesize heme. ④ key enzyme: ALA synthase (coenzyme: pyridoxal phosphate).
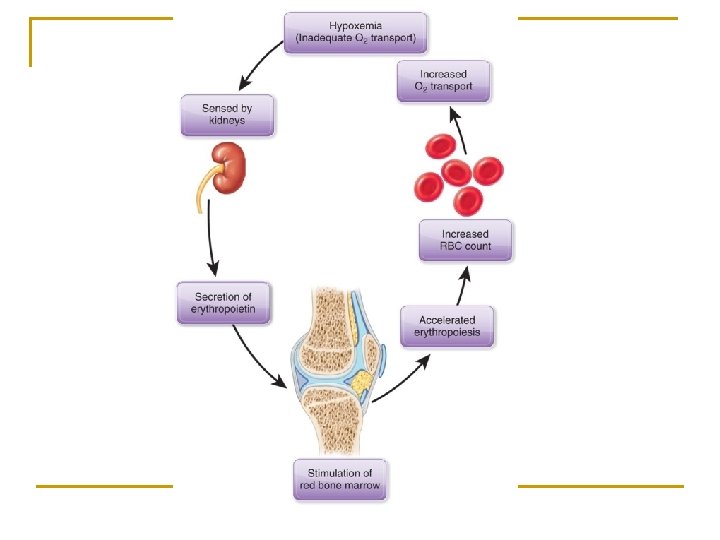
2) ALA dehydrase and ferrochelatase They are sensitive to heavy metal, such as plumbum. So plumbum toxicosis can lead to inhibition of heme synthesis and anemia. / iron-deficiency anemia 3) Erythropoietin (EPO) (Hypoxia, RBC↓) It is the major regulator of RBC generation. EPO is synthesized by the kidney and is released into the bloodstream. It combines with progenitors of RBC via a specific receptor. *EPO promotes synthesis of heme and Hb. *EPO promotes proliferation and differentiation of RBC progenitors.
Porphyria (卟啉症) Disorders that arise from defects in the enzymes of heme biosynthesis are termed the porphyrias and it causes elevations of intermediates in heme synthesis in the serum and urine. The porphyrias are both inherited and acquired disorders in heme synthesis.
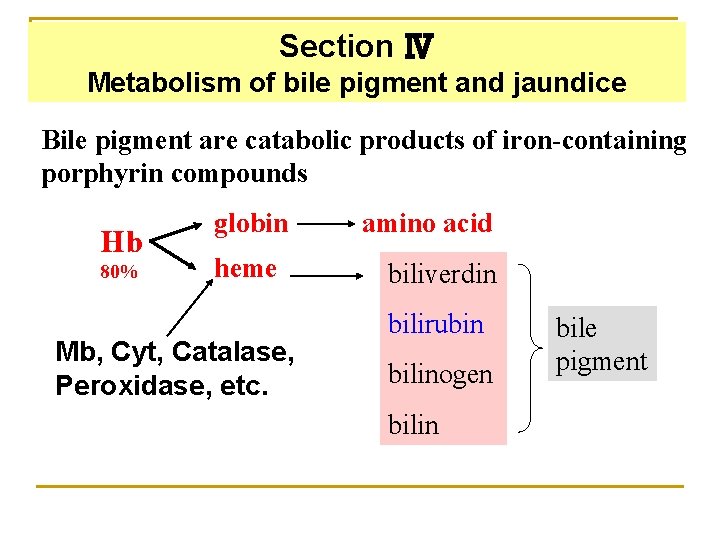
Section Ⅳ Metabolism of bile pigment and jaundice Bile pigment are catabolic products of iron-containing porphyrin compounds Hb 80% globin heme Mb, Cyt, Catalase, Peroxidase, etc. amino acid biliverdin bilirubin bilinogen bilin bile pigment
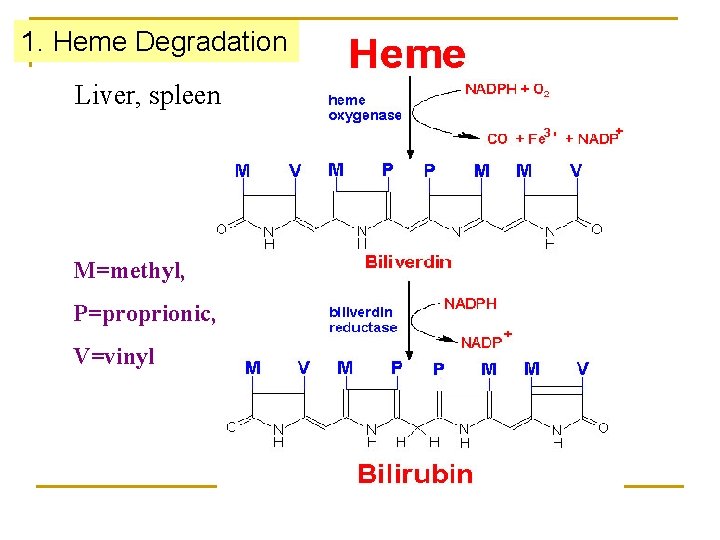
1. Heme Degradation Liver, spleen M=methyl, P=proprionic, V=vinyl
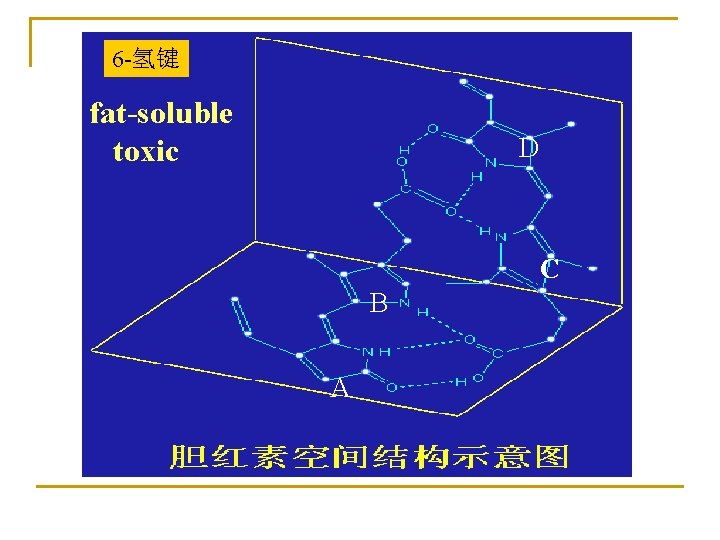
6 -氢键 fat-soluble toxic D C B A
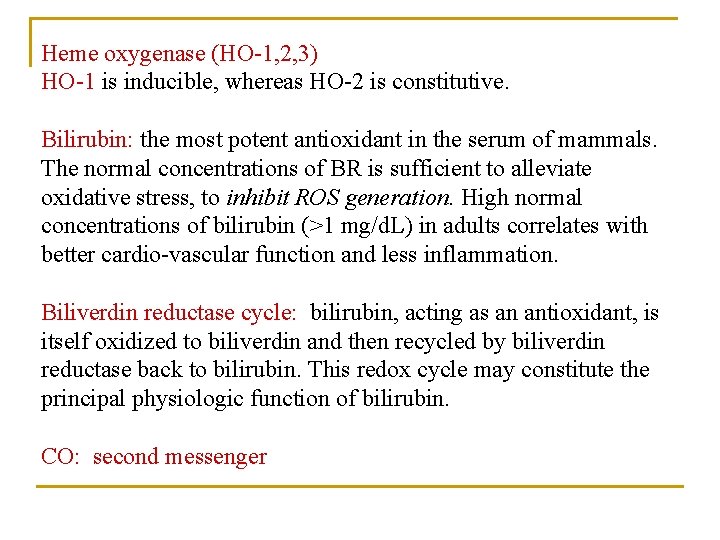
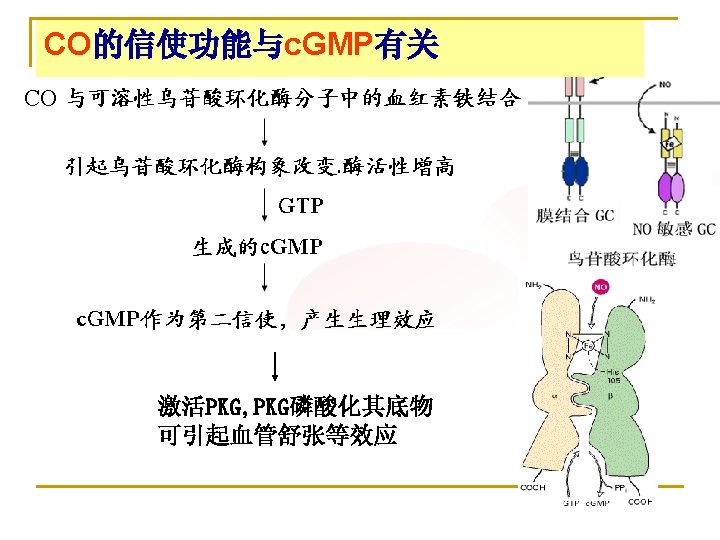
Heme oxygenase (HO-1, 2, 3) HO-1 is inducible, whereas HO-2 is constitutive. Bilirubin: the most potent antioxidant in the serum of mammals. The normal concentrations of BR is sufficient to alleviate oxidative stress, to inhibit ROS generation. High normal concentrations of bilirubin (>1 mg/d. L) in adults correlates with better cardio-vascular function and less inflammation. Biliverdin reductase cycle: bilirubin, acting as an antioxidant, is itself oxidized to biliverdin and then recycled by biliverdin reductase back to bilirubin. This redox cycle may constitute the principal physiologic function of bilirubin. CO: second messenger
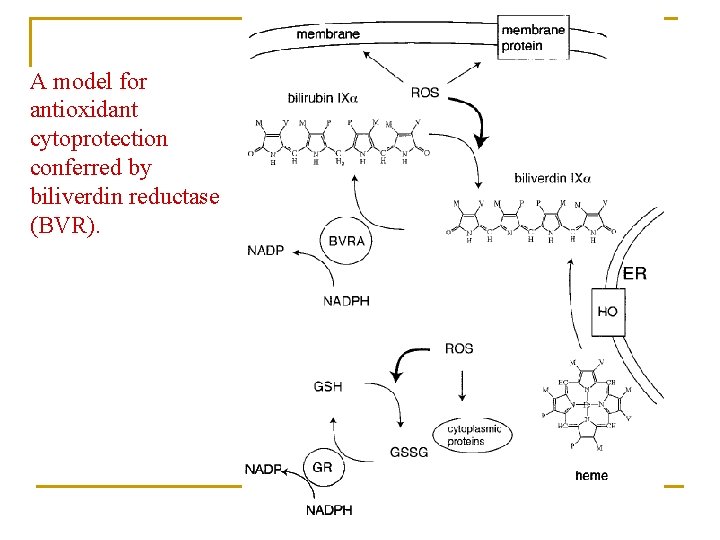
A model for antioxidant cytoprotection conferred by biliverdin reductase (BVR).
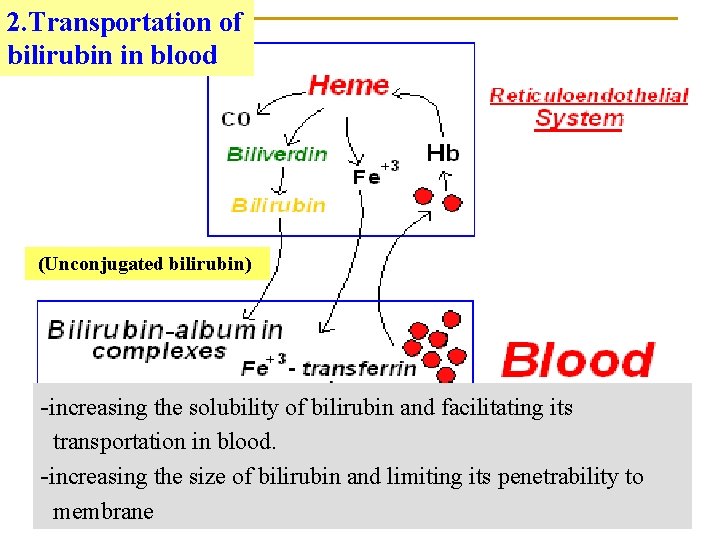
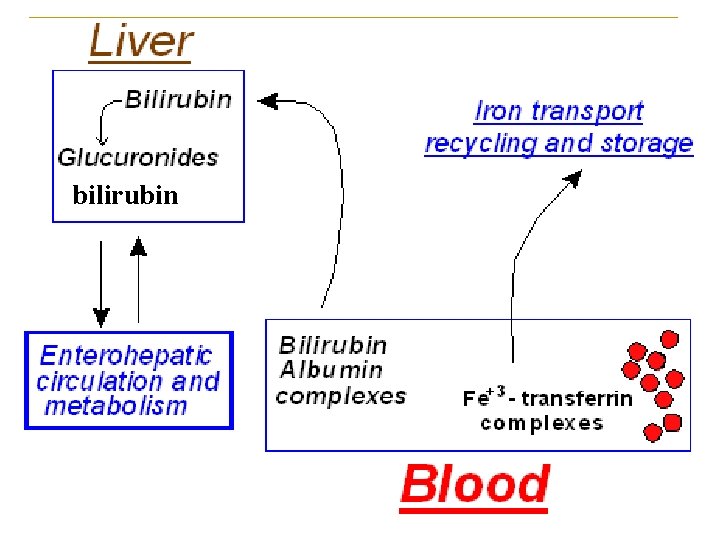
2. Transportation of bilirubin in blood (Unconjugated bilirubin) -increasing the solubility of bilirubin and facilitating its transportation in blood. -increasing the size of bilirubin and limiting its penetrability to membrane
bilirubin
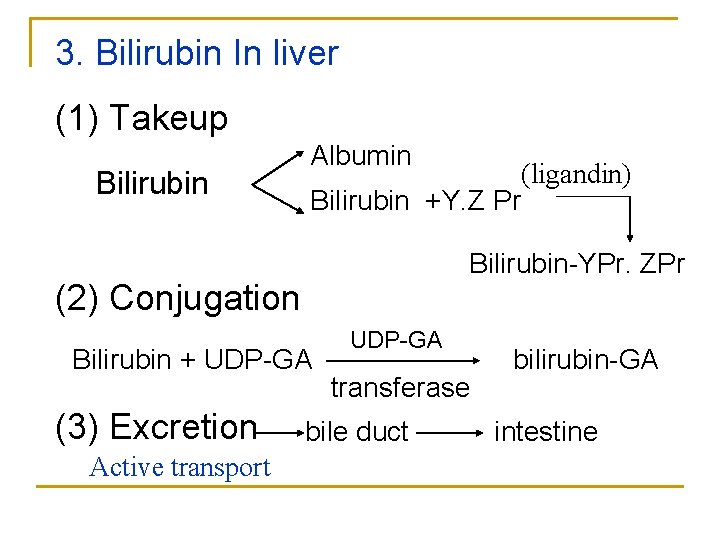
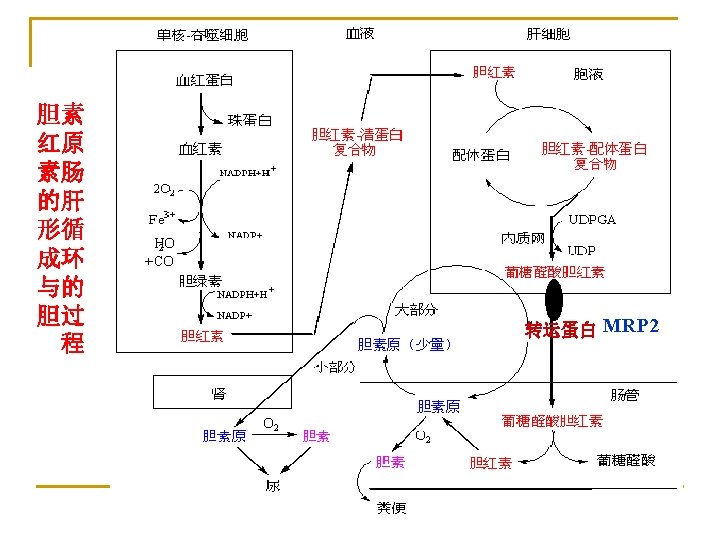
3. Bilirubin In liver (1) Takeup Bilirubin Albumin Bilirubin +Y. Z Pr Bilirubin-YPr. ZPr (2) Conjugation Bilirubin + UDP-GA (3) Excretion Active transport (ligandin) UDP-GA transferase bile duct bilirubin-GA intestine
bilirubin diglucuronide
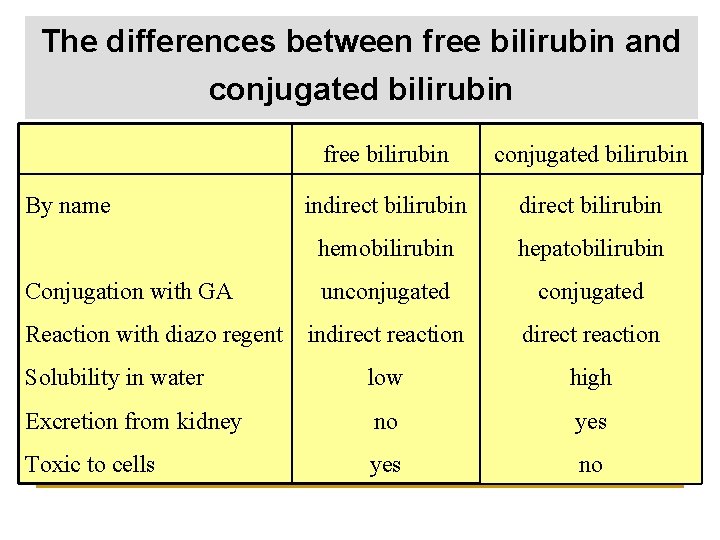
The differences between free bilirubin and conjugated bilirubin free bilirubin conjugated bilirubin indirect bilirubin hemobilirubin hepatobilirubin unconjugated indirect reaction Solubility in water low high Excretion from kidney no yes Toxic to cells yes no By name Conjugation with GA Reaction with diazo regent
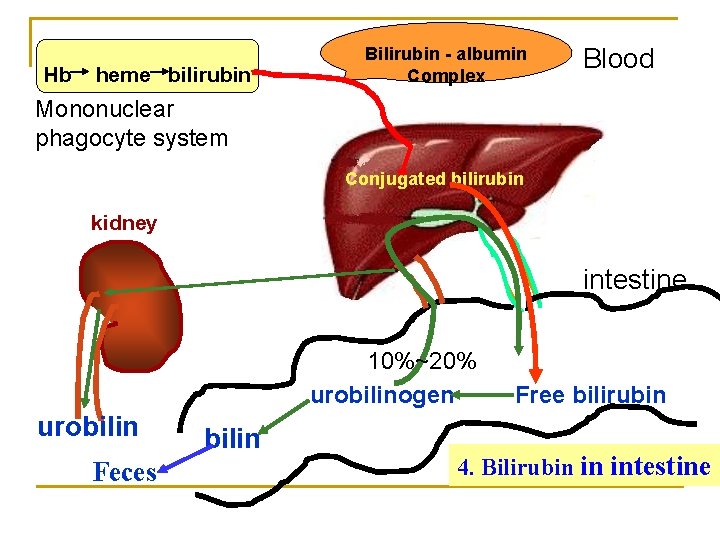
Hb heme bilirubin Bilirubin - albumin Complex Blood Mononuclear phagocyte system Conjugated bilirubin kidney intestine 10%~20% urobilinogen urobilin Feces bilin Free bilirubin 4. Bilirubin in intestine
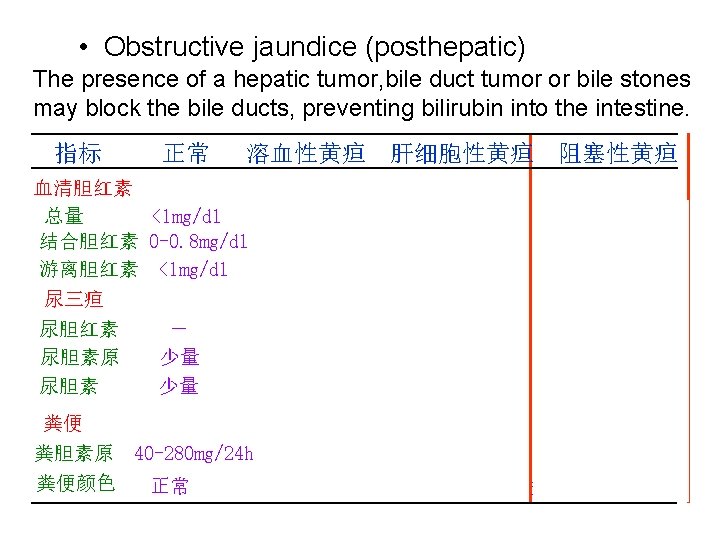
5. Serum bilirubin and jaundice • Normal serum bilirubin is 0. 2~0. 9 mg/dl 1/5 conjugated bilirubin and 4/5 free bilirubin • Accumulation of bilirubin in the blood leads to jaundice (>2 mg/dl) • 3 types of jaundice - Hemolytic jaundice - Hepatocellular jaundice - Obstructive jaundice
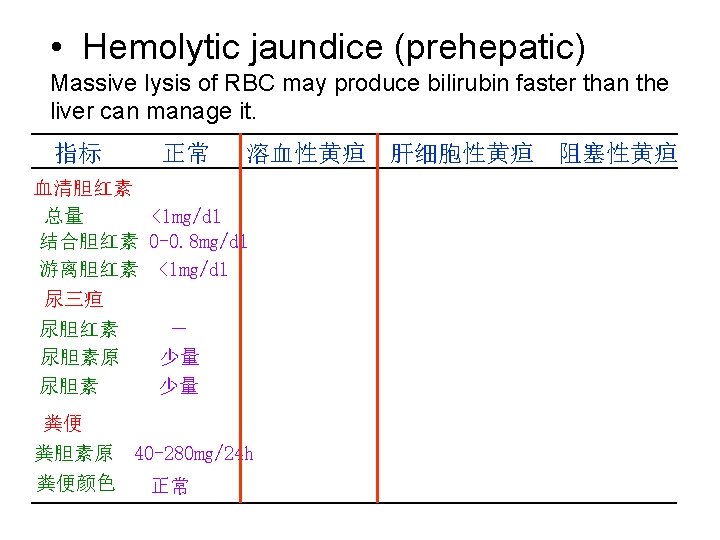
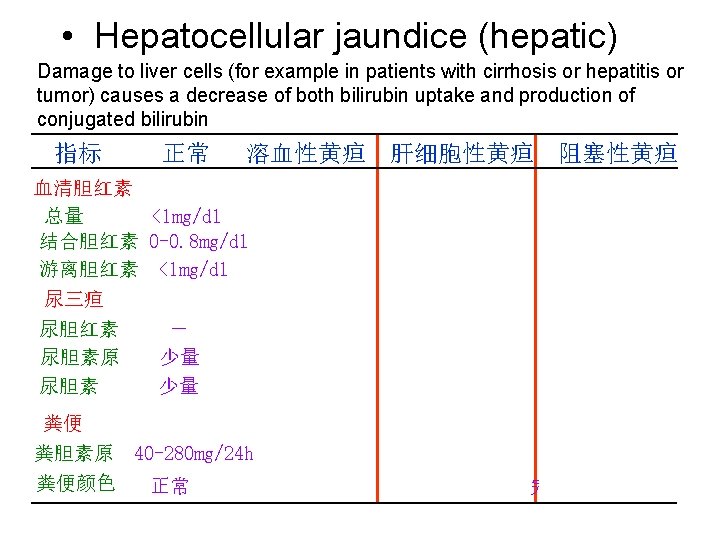
• Hepatocellular jaundice (hepatic) Damage to liver cells (for example in patients with cirrhosis or hepatitis or tumor) causes a decrease of both bilirubin uptake and production of conjugated bilirubin 指标 正常 溶血性黄疸 肝细胞性黄疸 阻塞性黄疸 血清胆红素 总量 <1 mg/dl 结合胆红素 0 -0. 8 mg/dl 游离胆红素 <1 mg/dl 尿三疸 尿胆红素 尿胆素原 尿胆素 - 少量 少量 粪便 粪胆素原 40 -280 mg/24 h 粪便颜色 正常 >1 mg/dl - ++ 不一定 >1 mg/dl ++ 不一定 -或微量 深 变浅或正常 完全阻塞时陶土色
Key terms 1. Biotransformation: 2. Enterohepatic circulation of bile acids:
- Slides: 59