Metabolism Glycolysis Glycolysis 1897 Hans and Eduard Buchner

Metabolism: Glycolysis
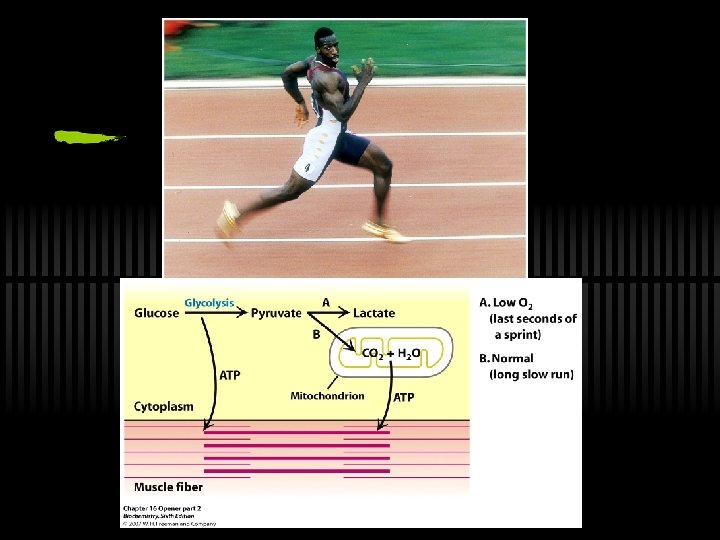

Glycolysis ü 1897: Hans and Eduard Buchner (Sucrose cell-free experiments; fermentation can take place outside of living cells) METABOLISM became simple chemistry ü Glycolysis: “Embden-Meyerhof pathway”

The all-important Glucose ü The only fuel the brain uses in nonstarvation conditions ü The only fuel red blood cells can use ü WHY? ü Evolutionary: probably available for primitive systems (from formaldehyde) ü Low tendency to glycosylate proteins, strong tendency to exist in ring form (recall: all equatorial!)
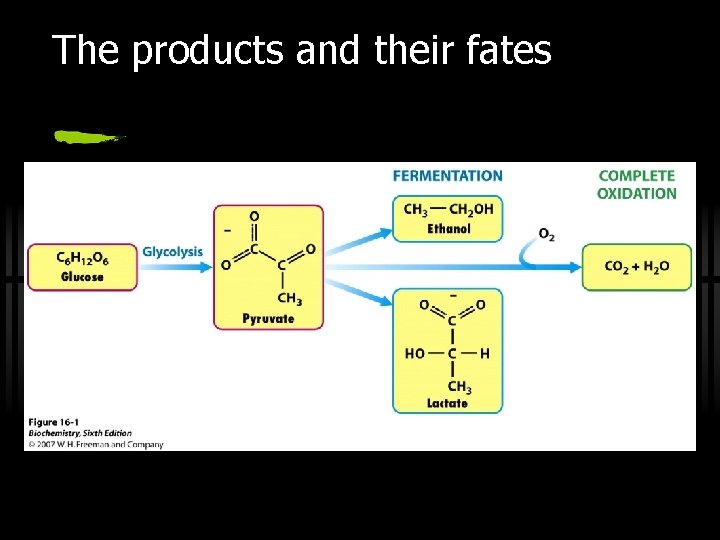
The products and their fates

Sidebar: Fermentation ü WHY? (lower energy yield) ü Oxygen is not required! ü OBLIGATE ANAEROBES (Cannot live with oxygen) ü FACULTATIVE ANAEROBES (Can live with or without oxygen)

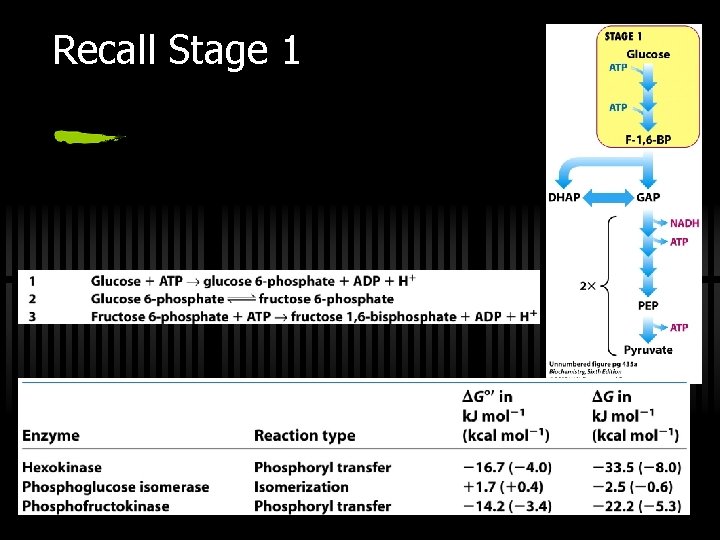
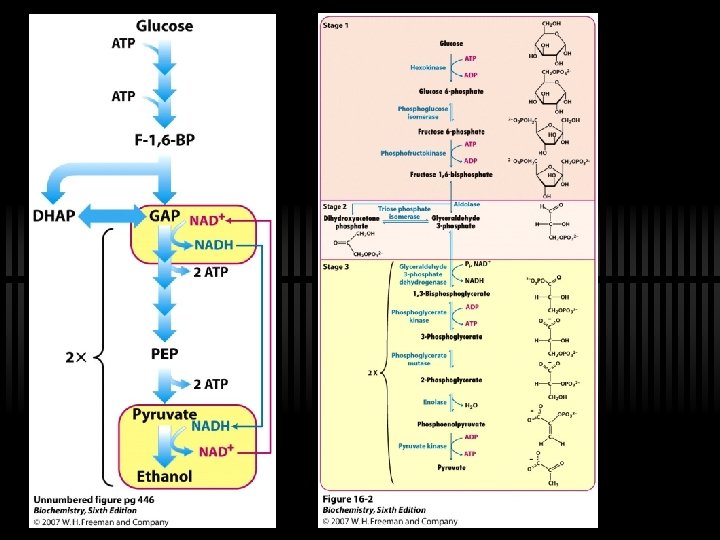
Glycolysis: a 10 -step pathway Three major stages 1. Glucose (G) to Fructose-1, 6 -bisphosphate (F 1, 6 -BP) ü Three steps, INVESTMENT of 2 ATP 2. F-1, 6 -BP to two three-carbon fragments ü Two component steps to glyceraldehde-3 phosphate (G 3 P) 3. G 3 P to Pyruvate ü Five steps, YIELD of 4 ATP

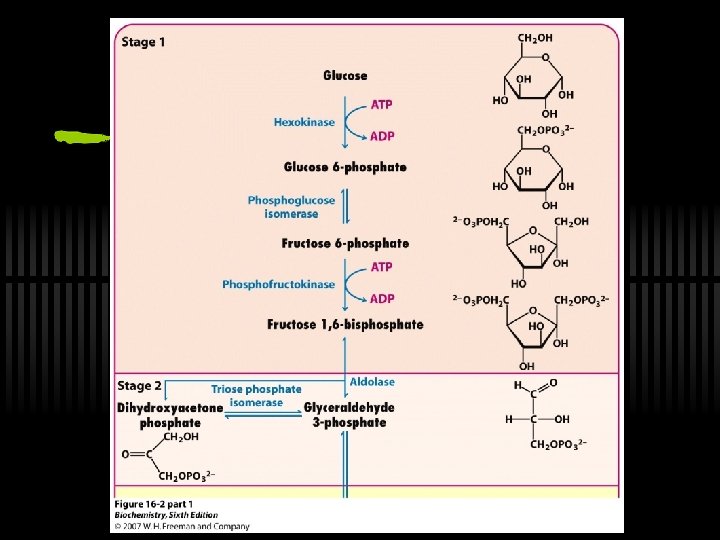
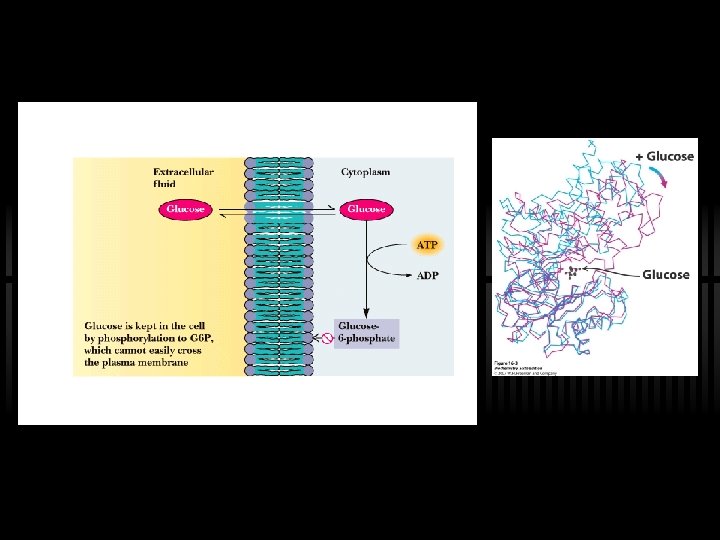
RXN 1: Phosphorylation of G The first reaction - phosphorylation of glucose ü Hexokinase or glucokinase ü This is a priming reaction - ATP is consumed here in order to get more later ü ATP makes the phosphorylation of glucose spontaneous ü Hexokinase (and glucokinase) act to phosphorylate glucose and keep it in the cell ü ATP is used, thus FIRST PRIMING REACTION G large, negative

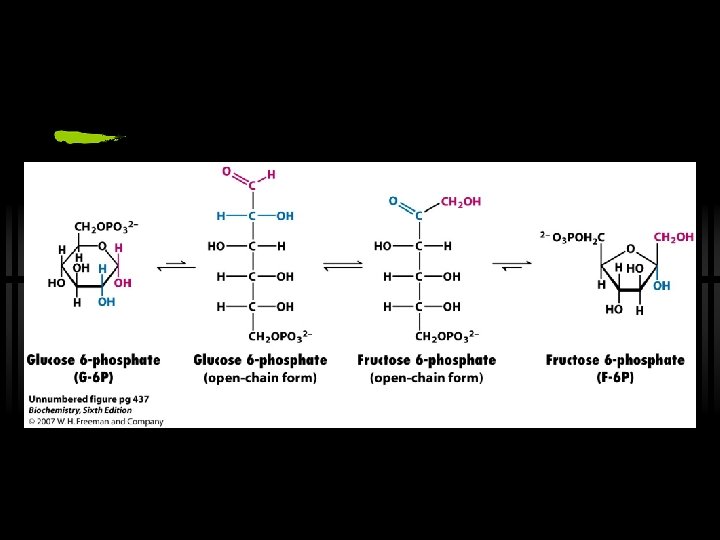
RXN 2: G 6 P to F 6 P ü Isomerization of glucose-6 -phosphate to fructose-6 -phosphate ü By phosphoglucose isomerase ü Why does this reaction occur? ? ü next step (phosphorylation at C-1) would be tough for hemiacetal -OH, but easy for primary -OH ü isomerization activates C-3 for cleavage in aldolase reaction
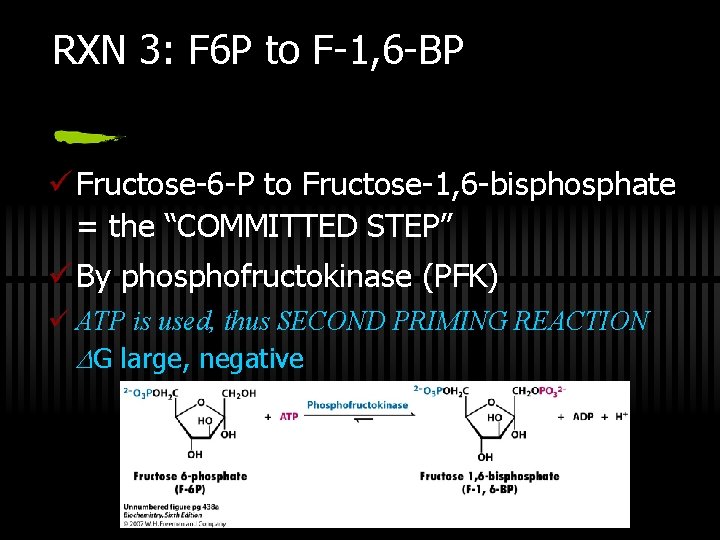
RXN 3: F 6 P to F-1, 6 -BP ü Fructose-6 -P to Fructose-1, 6 -bisphosphate = the “COMMITTED STEP” ü By phosphofructokinase (PFK) ü ATP is used, thus SECOND PRIMING REACTION G large, negative
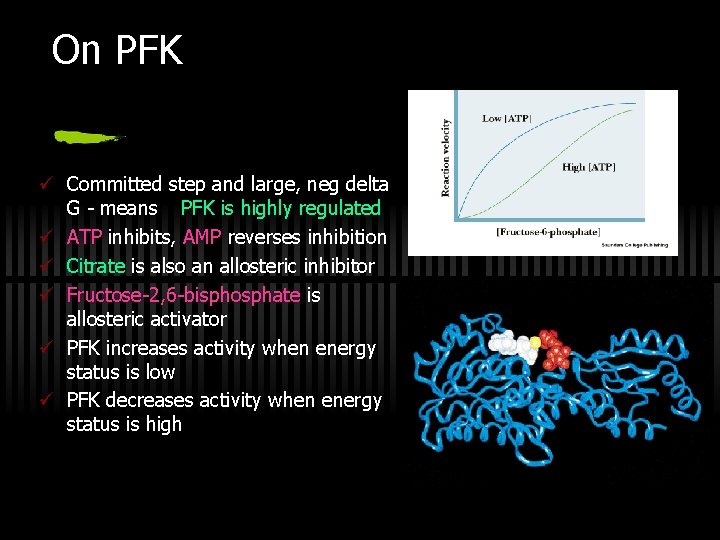
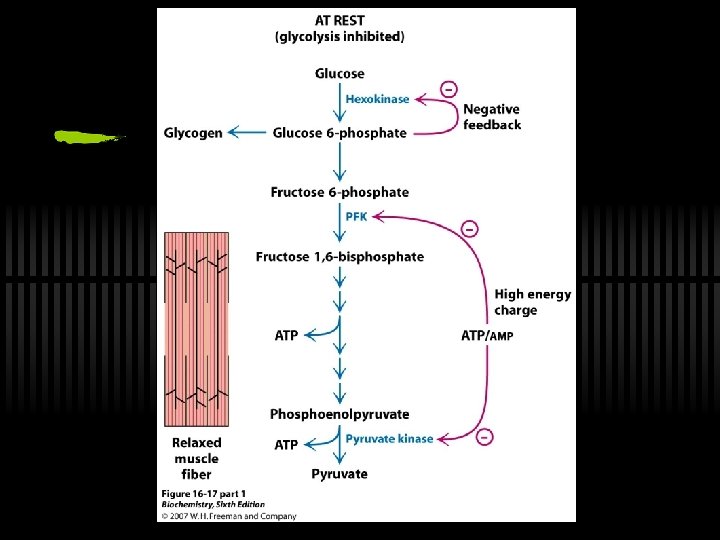
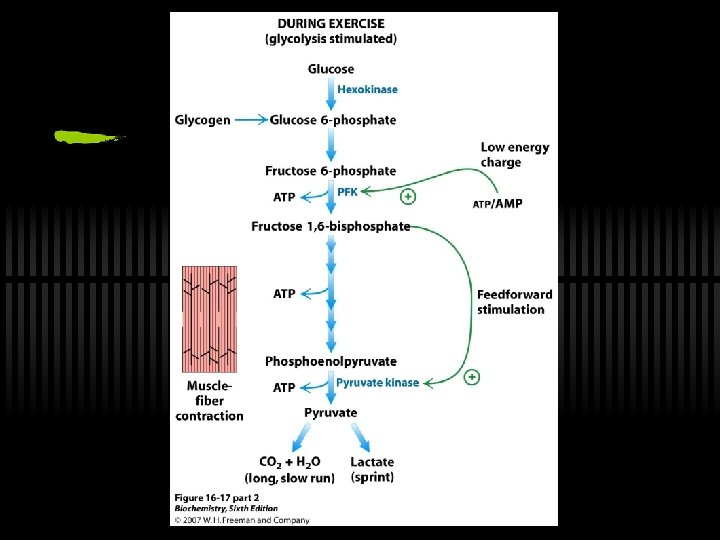
On PFK ü Committed step and large, neg delta G - means PFK is highly regulated ü ATP inhibits, AMP reverses inhibition ü Citrate is also an allosteric inhibitor ü Fructose-2, 6 -bisphosphate is allosteric activator ü PFK increases activity when energy status is low ü PFK decreases activity when energy status is high
Recall Stage 1
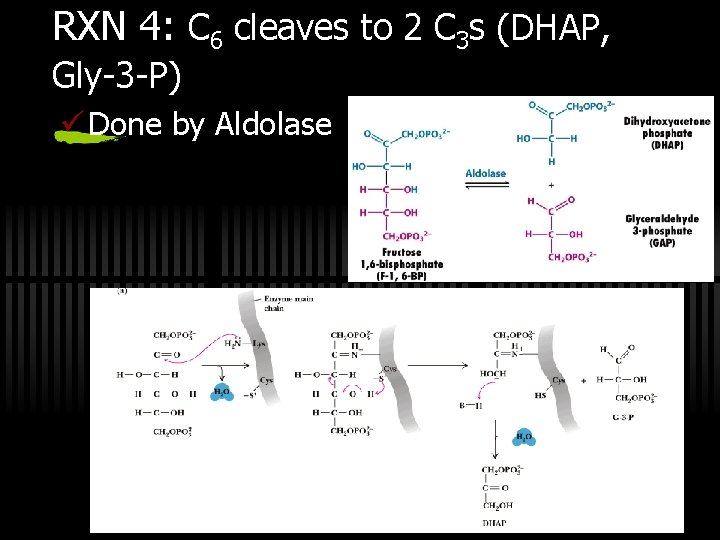
RXN 4: C 6 cleaves to 2 C 3 s (DHAP, Gly-3 -P) ü Done by Aldolase
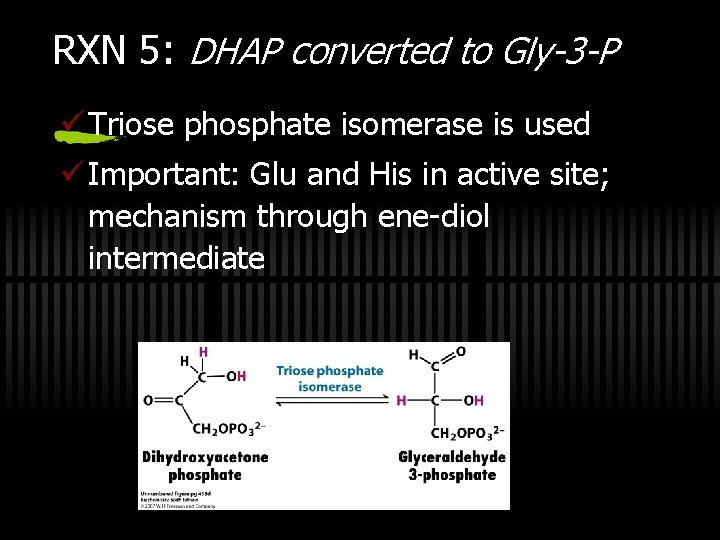
RXN 5: DHAP converted to Gly-3 -P ü Triose phosphate isomerase is used ü Important: Glu and His in active site; mechanism through ene-diol intermediate
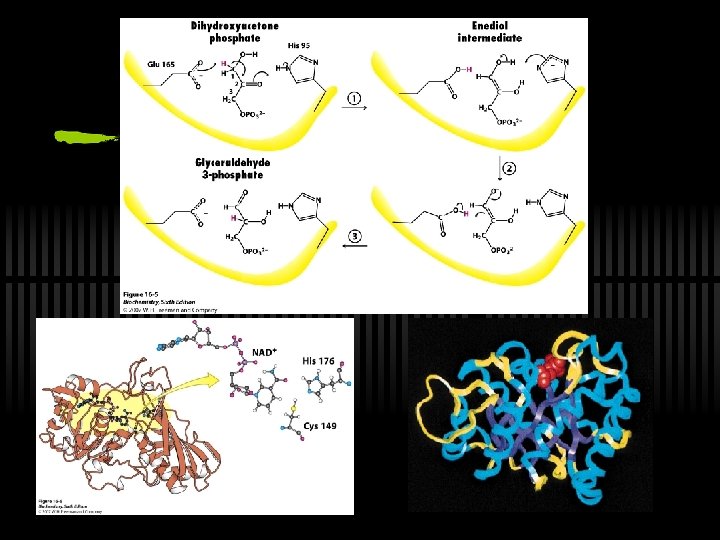
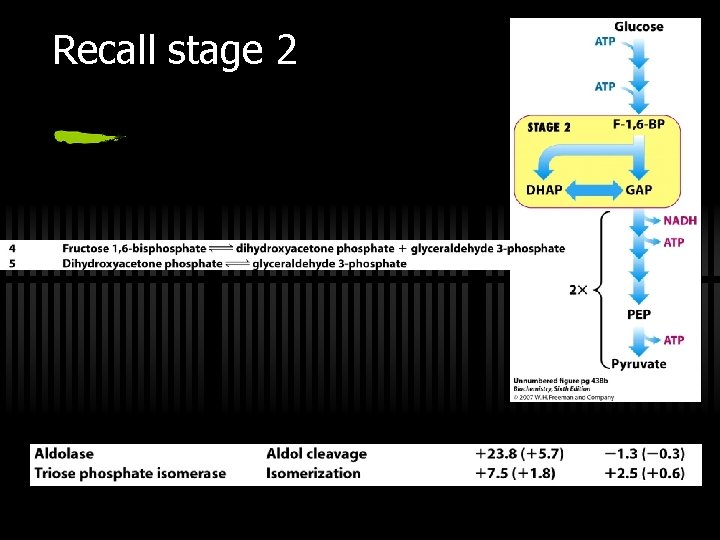
Recall stage 2

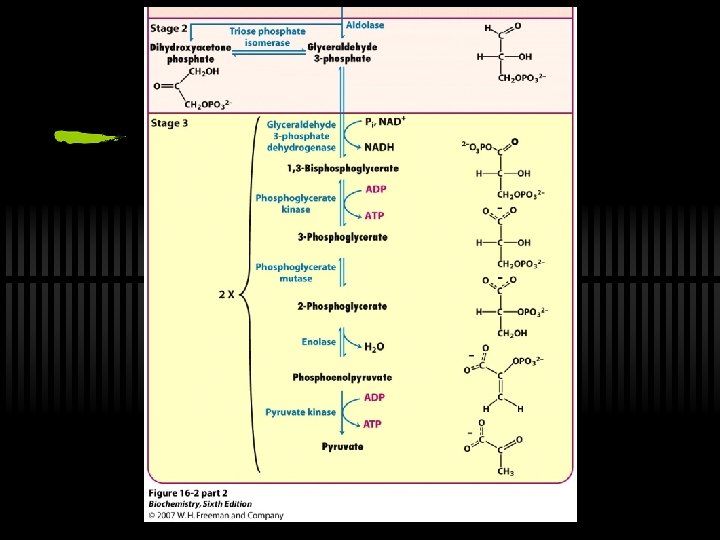
So far… ü We’ve USED UP 2 ATP molecules to process 1 glucose molecule ü We are left with 2 G 3 P now ü Time for energy payback, thus STAGE 3! ü Recall that stage 3 happens in parallel to the two G 3 P molecules
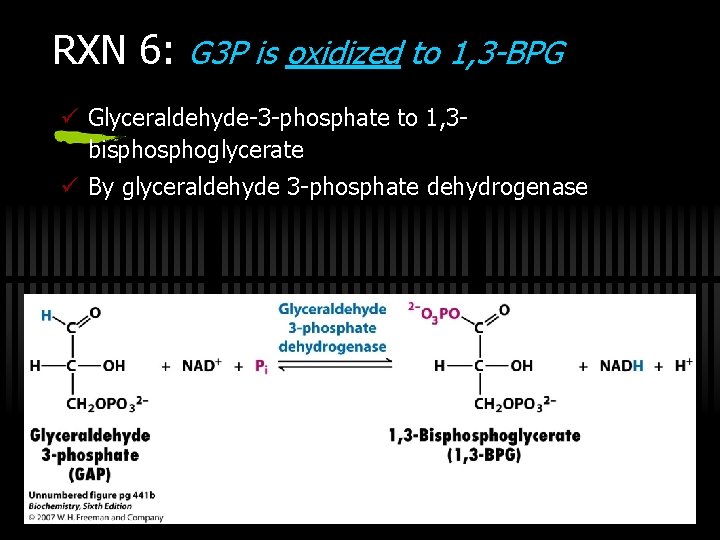
RXN 6: G 3 P is oxidized to 1, 3 -BPG ü Glyceraldehyde-3 -phosphate to 1, 3 bisphoglycerate ü By glyceraldehyde 3 -phosphate dehydrogenase
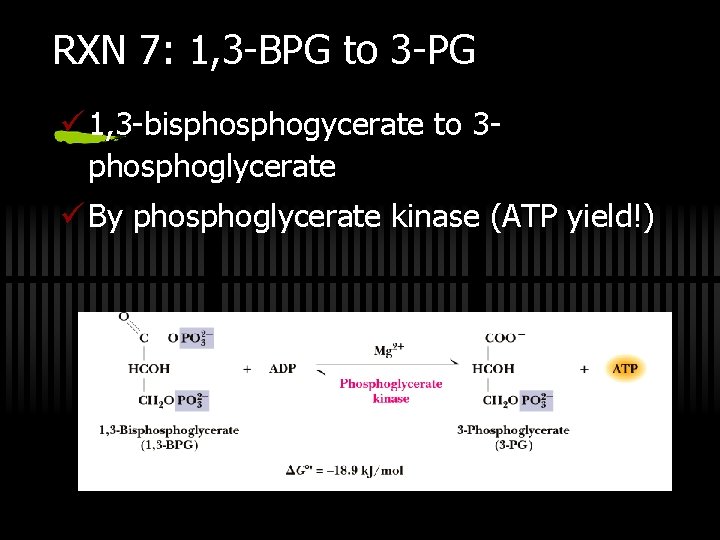
RXN 7: 1, 3 -BPG to 3 -PG ü 1, 3 -bisphogycerate to 3 phosphoglycerate ü By phosphoglycerate kinase (ATP yield!)
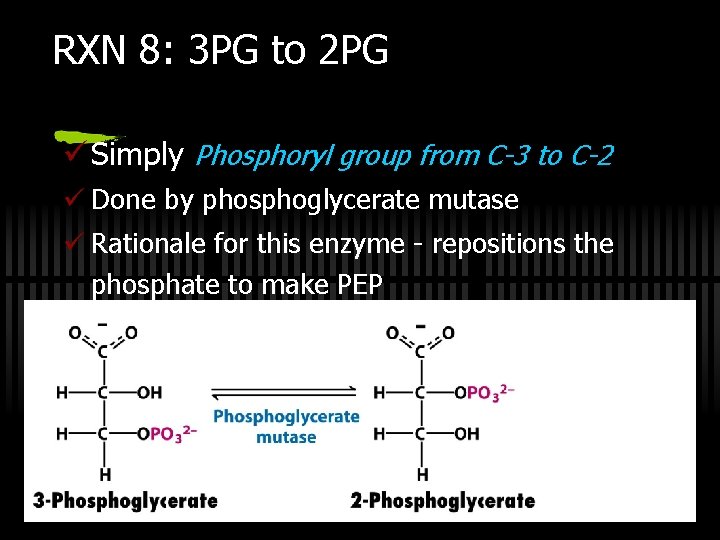
RXN 8: 3 PG to 2 PG ü Simply Phosphoryl group from C-3 to C-2 ü Done by phosphoglycerate mutase ü Rationale for this enzyme - repositions the phosphate to make PEP
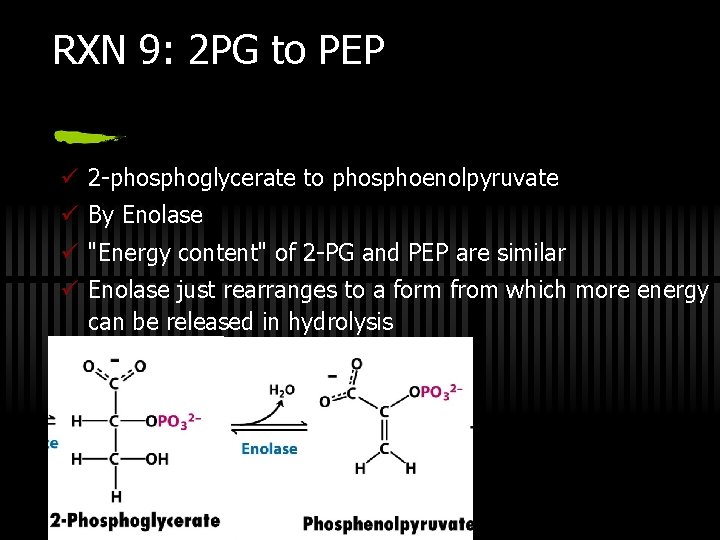
RXN 9: 2 PG to PEP ü 2 -phosphoglycerate to phosphoenolpyruvate ü By Enolase ü "Energy content" of 2 -PG and PEP are similar ü Enolase just rearranges to a form from which more energy can be released in hydrolysis
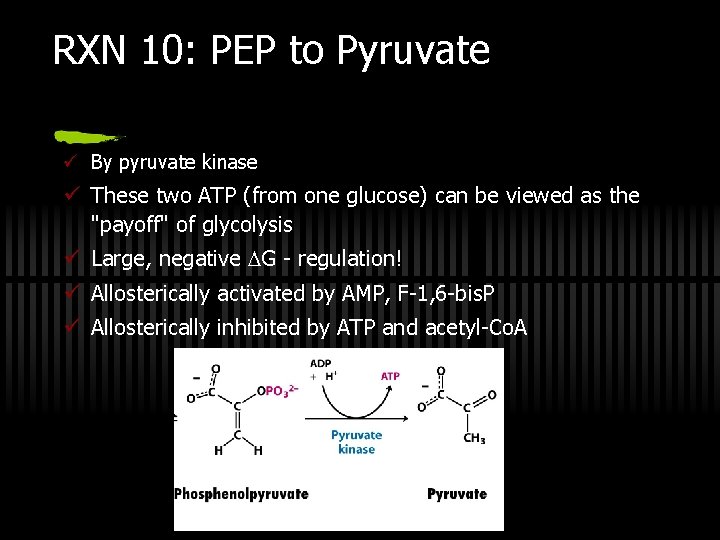
RXN 10: PEP to Pyruvate ü By pyruvate kinase ü These two ATP (from one glucose) can be viewed as the "payoff" of glycolysis ü Large, negative G - regulation! ü Allosterically activated by AMP, F-1, 6 -bis. P ü Allosterically inhibited by ATP and acetyl-Co. A
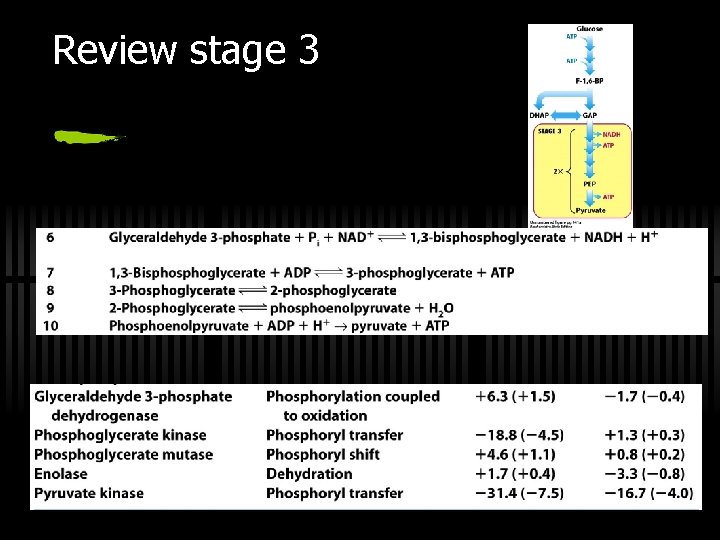
Review stage 3
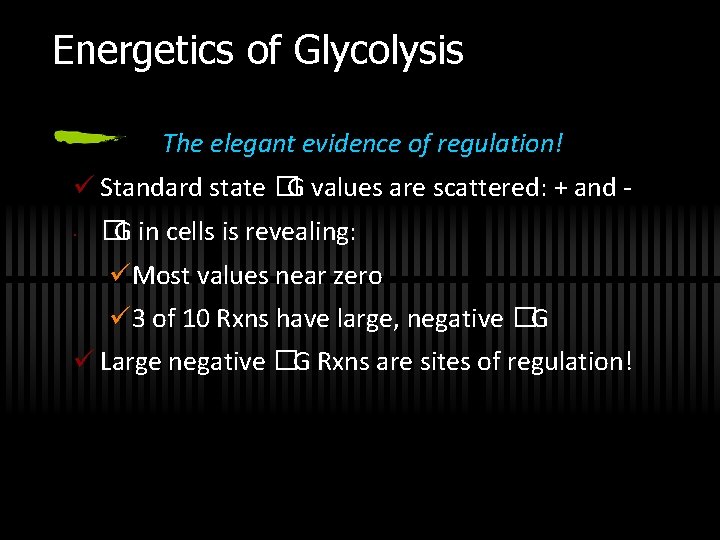
Energetics of Glycolysis The elegant evidence of regulation! ü Standard state �G values are scattered: + and · �G in cells is revealing: üMost values near zero ü 3 of 10 Rxns have large, negative �G ü Large negative �G Rxns are sites of regulation!

GLYCOLYSIS NET ü Glucose + 2 Pi + 2 ADP + 2 NAD+ -> 2 pyruvate + 2 ATP + 2 NADH + 2 H+ + 2 H 2 O

What Now? : The Fate of NADH and Pyruvate ü Aerobic or anaerobic, that is the question. ü NADH is energy - two possible fates: ü If O 2 is available, NADH is re-oxidized in the electron transport pathway, making ATP in oxidative phosphorylation ü In anaerobic conditions, NADH is re-oxidized by lactate dehydrogenase (LDH), providing additional NAD+ for more glycolysis

The Fate of NADH and Pyruvate ü Pyruvate is also energy - two general possible fates (AEROBIC/ANAEROBIC): ü aerobic: citric acid cycle ü anaerobic: to lactate (lactic acid fermentation) or to ethanol (alcoholic fermentation)
- Slides: 35