Membrane Biophysics 102014 Anion Channels Selectivity gradient Plasma

Membrane Biophysics 10/2014

Anion Channels • Selectivity gradient • Plasma membrane; intracellular organelle membranes • Set Resting Potential • Provide transport, excitability and inhibition • Activated by Hyperpolarization • Cell Swelling • p. H Levels
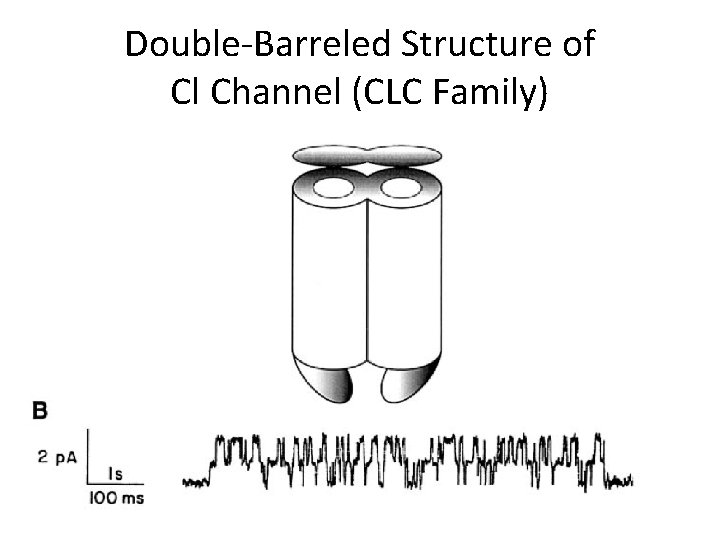
Double-Barreled Structure of Cl Channel (CLC Family)
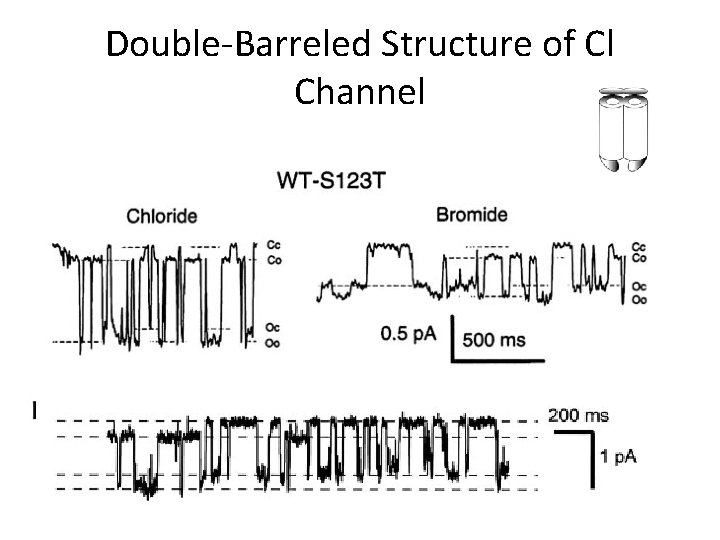
Double-Barreled Structure of Cl Channel
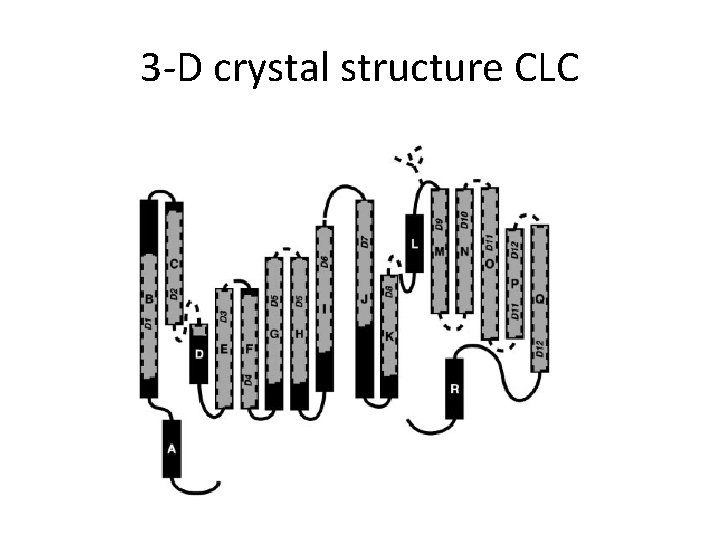
3 -D crystal structure CLC
CLC Family Members • • • CLC-0; 1 st to be studied CLC-1; Skeletal muscle CLC-2; Broadly expressed CLC-K; Kidney epithelia and inner ear cells CLC-3; Intracellular, synaptic vesicles and organelles CLC-4; Vesicular channel CLC-5; Endosomal channel CLC-6; Intracellular channel CLC-7; Lysosomal channel
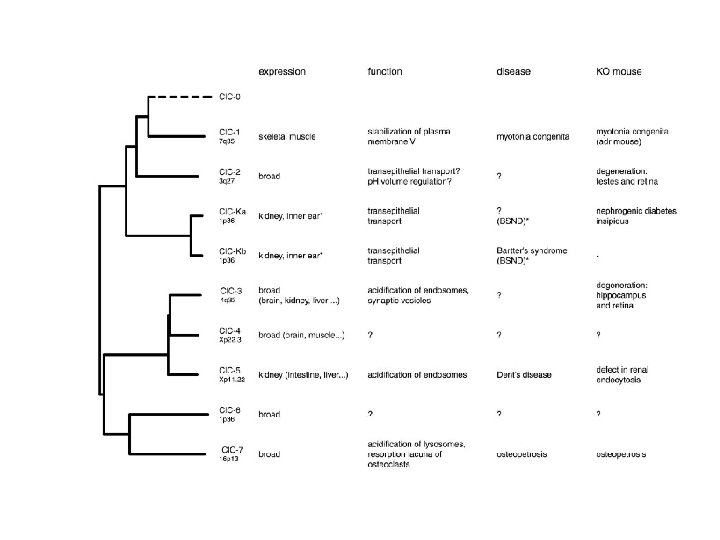
CLC-1 • Activity dependent • 70 -80% RM • Skeletal muscle
CLC-2 • Important for cell-to-cell communication and survival in early development • Activated by: – Hyperpolarization – Cell swelling – Acidic p. H
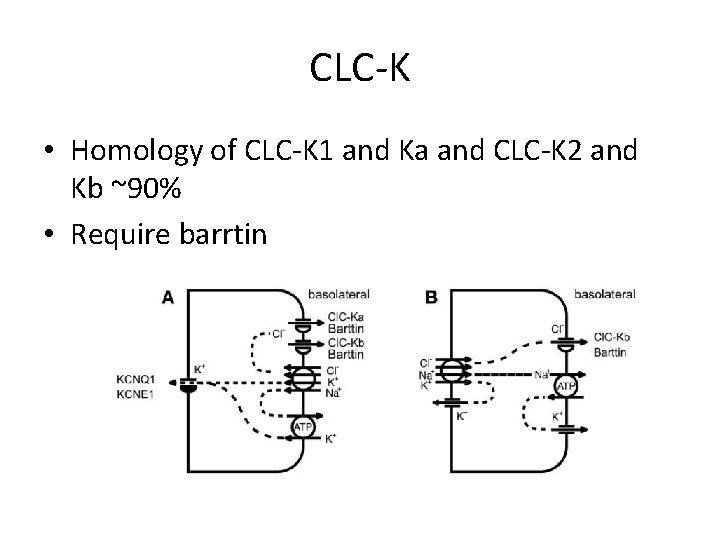
CLC-K • Homology of CLC-K 1 and Ka and CLC-K 2 and Kb ~90% • Require barrtin
CLC-3 • Intracellular; endosomes and synaptic vesicles • Modulates Ca 2+ activated Cl- currents

CLC-4; CLC-5 • • Intracellular membrane Relatively mysterious Extreme outward rectification Inhibition by extracellular acidic p. H
Cystic Fibrosis Transmembrane Conductance Regulator • c. AMP activated • Expressed in apical membrane of many cell types • Several phosphorylated sites required to open channel • Regulates other ion channels

Swelling Activated Chloride Channels • ICl, swell • Moderate outward rectification • Likely 2 nd messenger, not mechanically activated (not time-dependent)
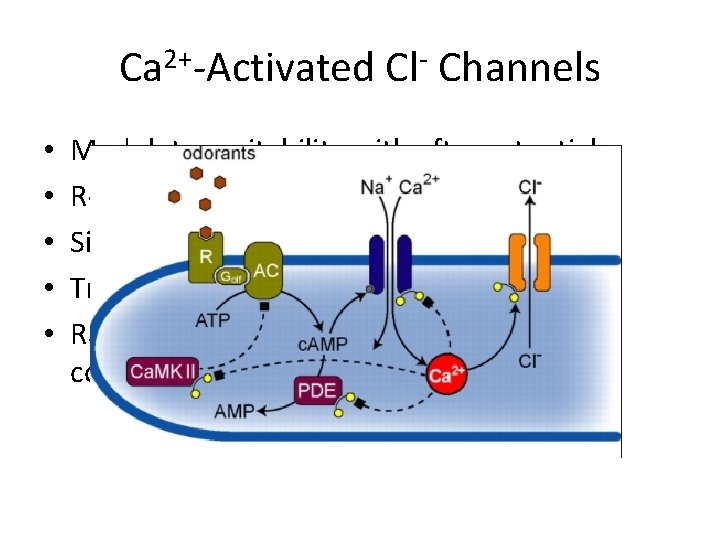
Ca 2+-Activated Cl- Channels • • • Modulate excitability with afterpotentials Regulate tonus of smooth muscles Signal transduction Transepithelial transport Range from 1 -70 p. S single-channel conductances

Intracellular Chloride Channels • Overexpression brings to PM • Little is known about the native tissue • Near dense-core vesicles

Ligand Gated Chloride Channels • Excitatory neurotransmitter binding in early development • Fast-inhibitory neurotransmitter binding – GABAA&C (brain), glycine (spinal cord)

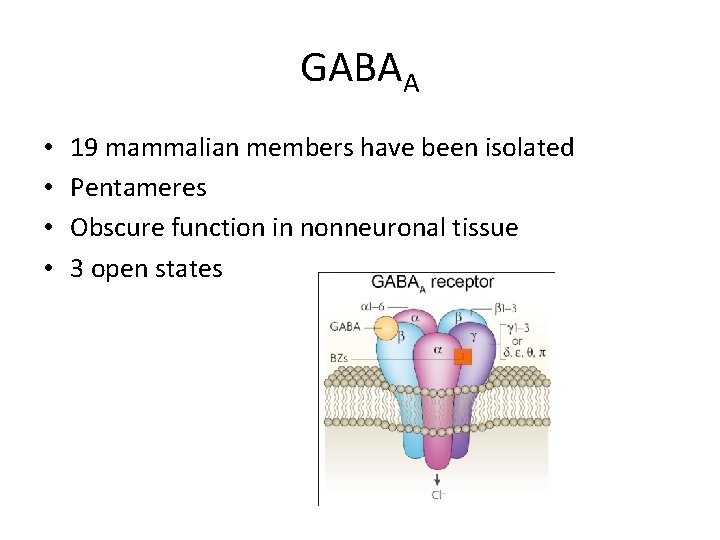
GABAA • • 19 mammalian members have been isolated Pentameres Obscure function in nonneuronal tissue 3 open states

GABAc • Higher sensitivity to GABA • Smaller currents • Do not desensitize
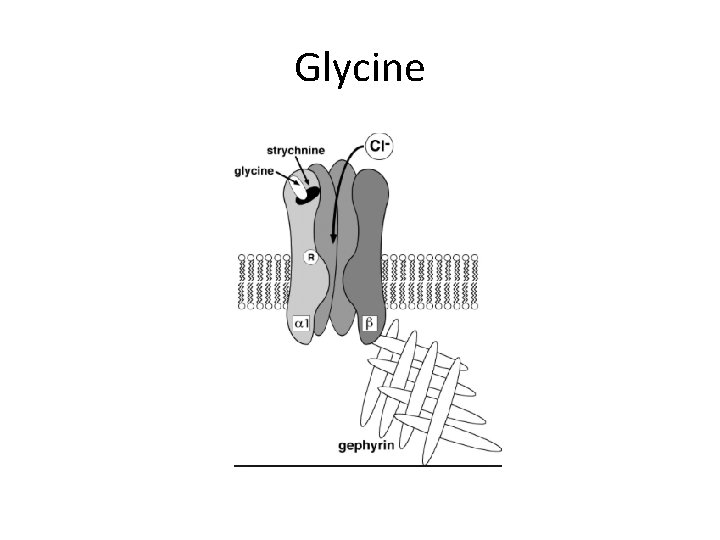
Glycine

CLC-1 Channels • CLC-1 contributes 70 -80% of the resting membrane conductance of skeletal muscles • CLC-1 mutations altering common gating cause myotonia congenita – genetic neuromuscular channelopathy in which an initiated muscle contraction fails to terminate • Bennetts and Parker used a Model of Cl-/H+ transport in a prokaryotic CLC CL-/H+ antiporter to model CLC-1

CLC Channels • Homodimers • Each subunit has its own separate, identical ion conducting pore • 2 Gates regulate channel acitivty – Protopore Gate, which regulates each individual pore – Common gate, which regulates both pore simultaneously Duran et al. 2010
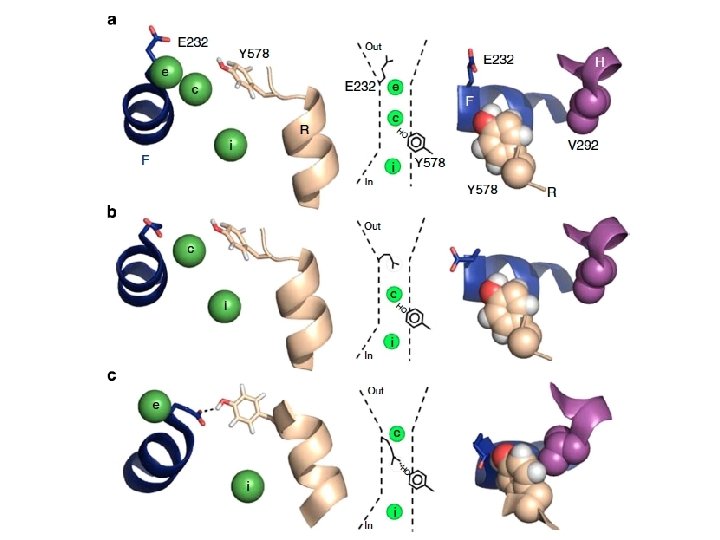
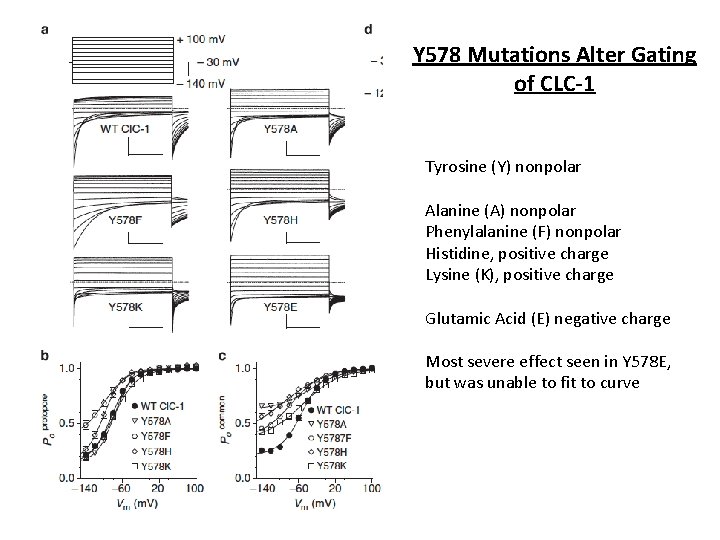
Y 578 Mutations Alter Gating of CLC-1 Tyrosine (Y) nonpolar Alanine (A) nonpolar Phenylalanine (F) nonpolar Histidine, positive charge Lysine (K), positive charge Glutamic Acid (E) negative charge Most severe effect seen in Y 578 E, but was unable to fit to curve
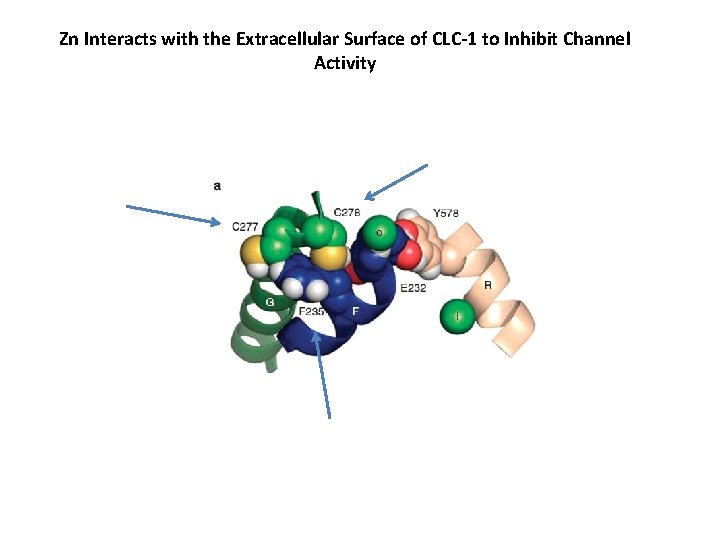
Zn Interacts with the Extracellular Surface of CLC-1 to Inhibit Channel Activity

Y 578 Mutations Alter CLC inhibition by Zn E 232, Glutamic Acid = negative charge Insensitive to Zn Y 578 A and Y 578 F are nonpolar Y 578 E is negatively charged Above mutations negate favorable interactions with E 232 Inhibited by Zn Y 578 K and Y 578 H have positive charged Above mutations promote favorable interactions with E 232
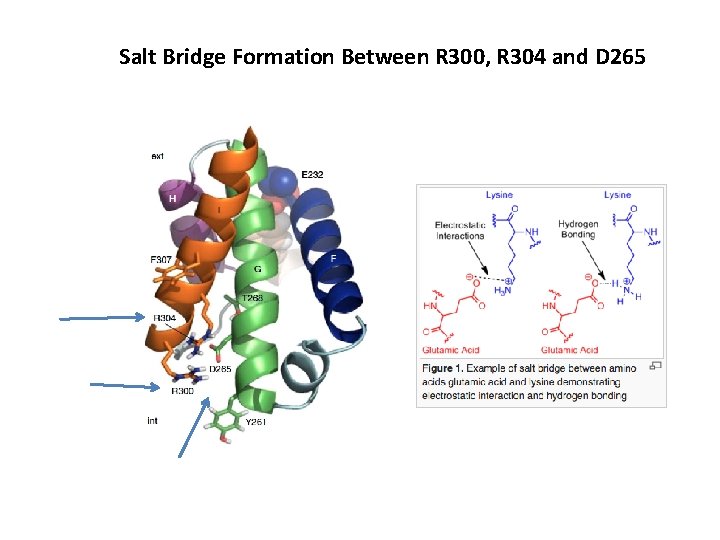
Salt Bridge Formation Between R 300, R 304 and D 265

How do Y 578 Mutants effect NAD + Inhibition of CLC-1 Channels?
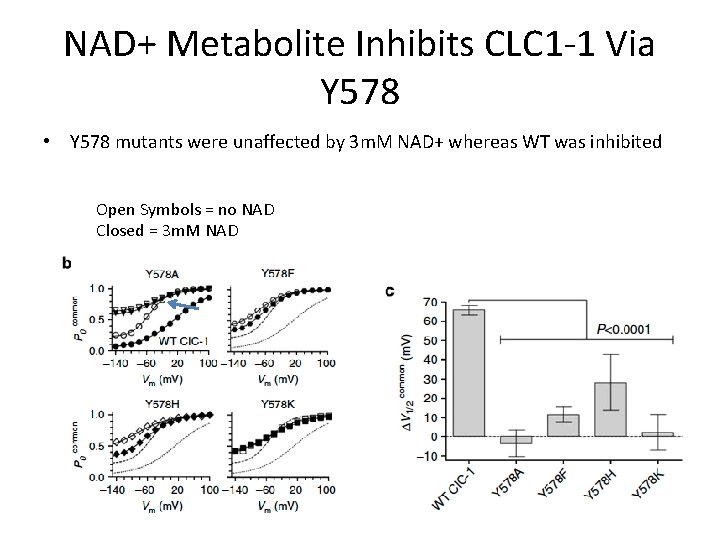
NAD+ Metabolite Inhibits CLC 1 -1 Via Y 578 • Y 578 mutants were unaffected by 3 m. M NAD+ whereas WT was inhibited Open Symbols = no NAD Closed = 3 m. M NAD

• Mutations to Y 578 alter CBS interactions with CLC-1
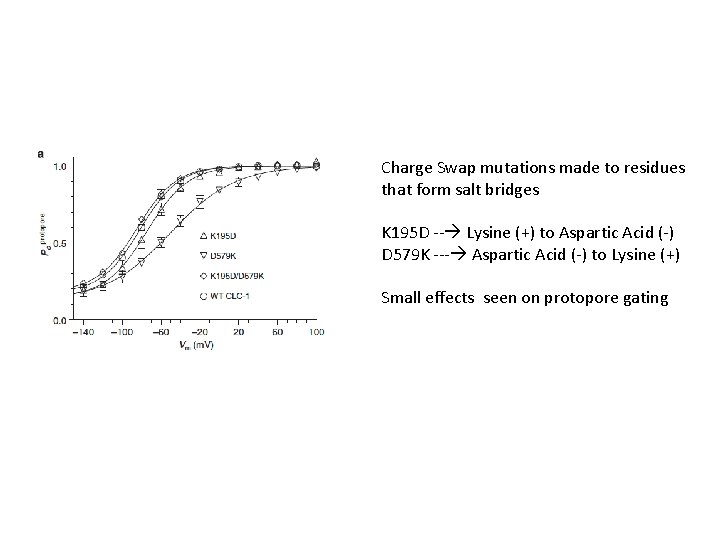
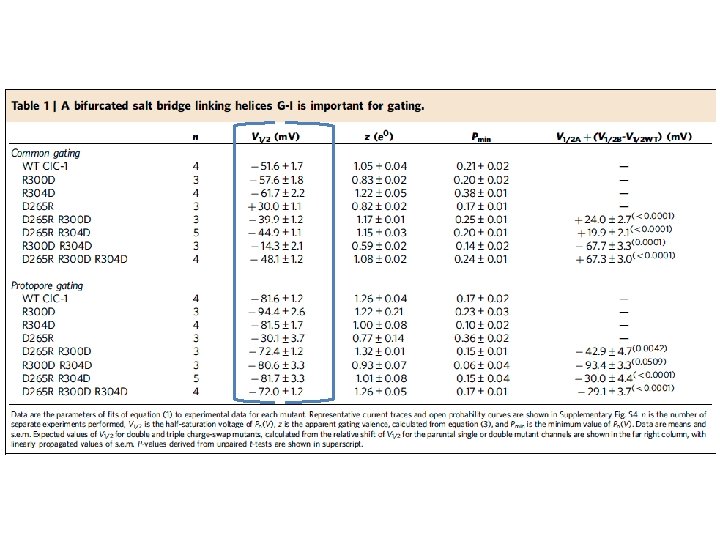
Charge Swap mutations made to residues that form salt bridges K 195 D -- Lysine (+) to Aspartic Acid (-) D 579 K --- Aspartic Acid (-) to Lysine (+) Small effects seen on protopore gating
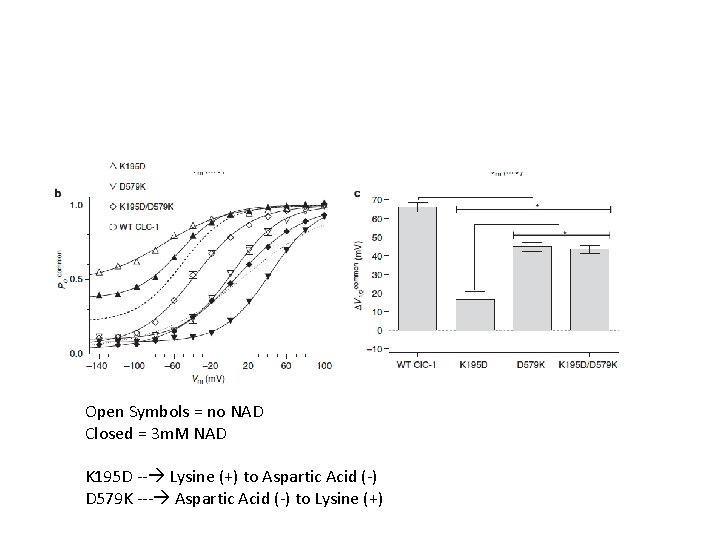
Open Symbols = no NAD Closed = 3 m. M NAD K 195 D -- Lysine (+) to Aspartic Acid (-) D 579 K --- Aspartic Acid (-) to Lysine (+)


Background
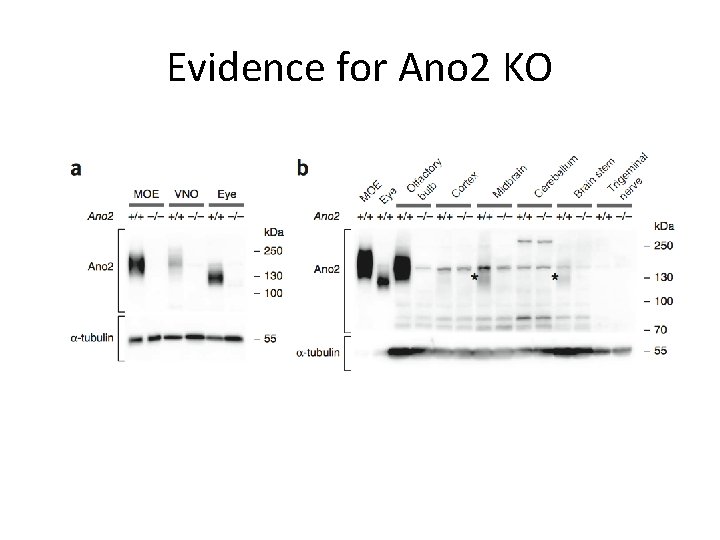
Evidence for Ano 2 KO

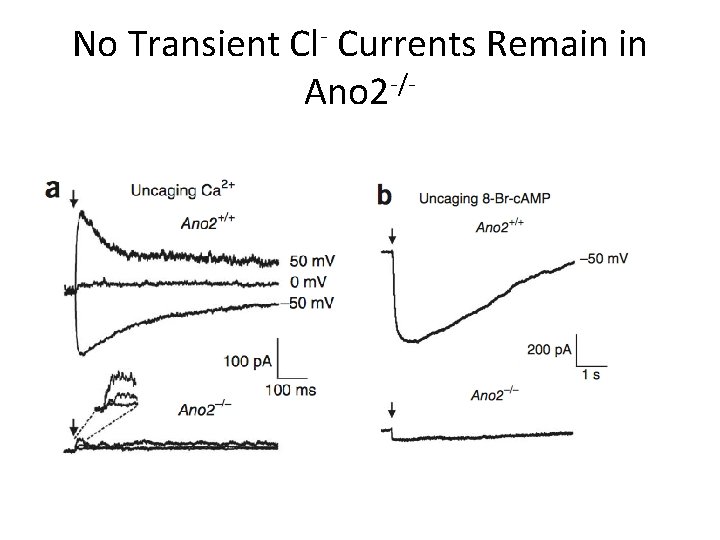
No Transient Cl- Currents Remain in Ano 2 -/-
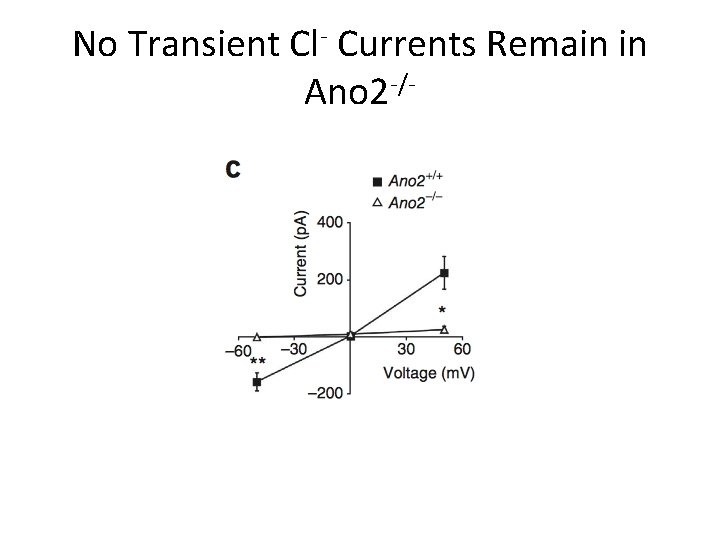
No Transient Cl- Currents Remain in Ano 2 -/-
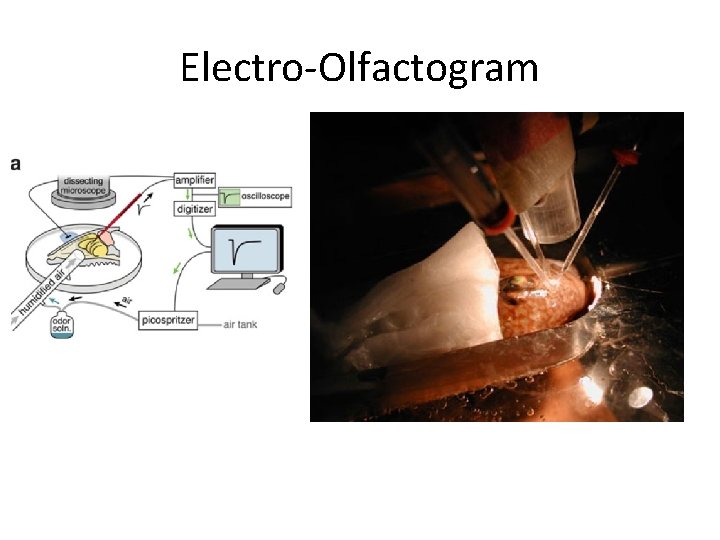
Electro-Olfactogram
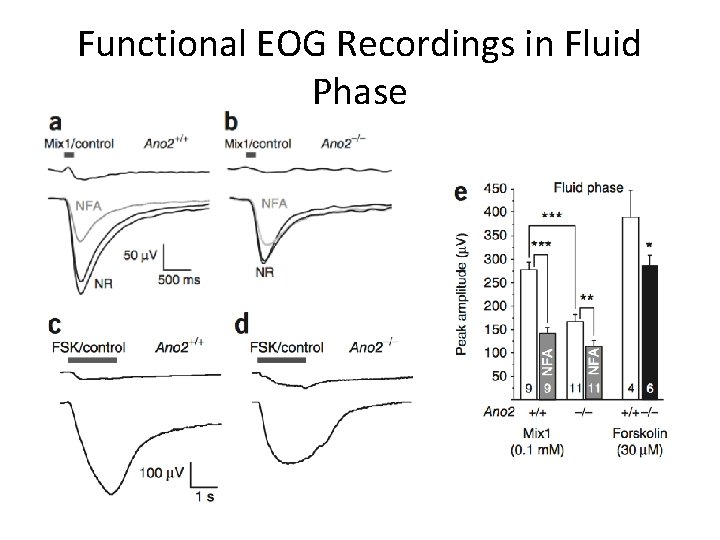
Functional EOG Recordings in Fluid Phase
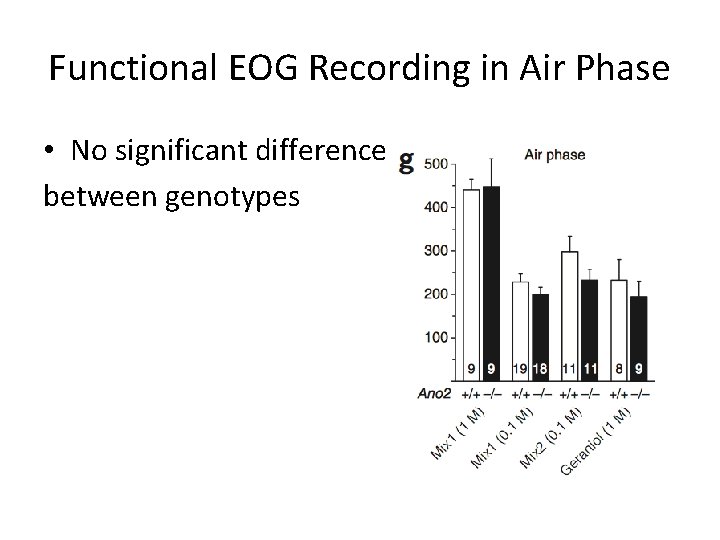
Functional EOG Recording in Air Phase • No significant difference between genotypes
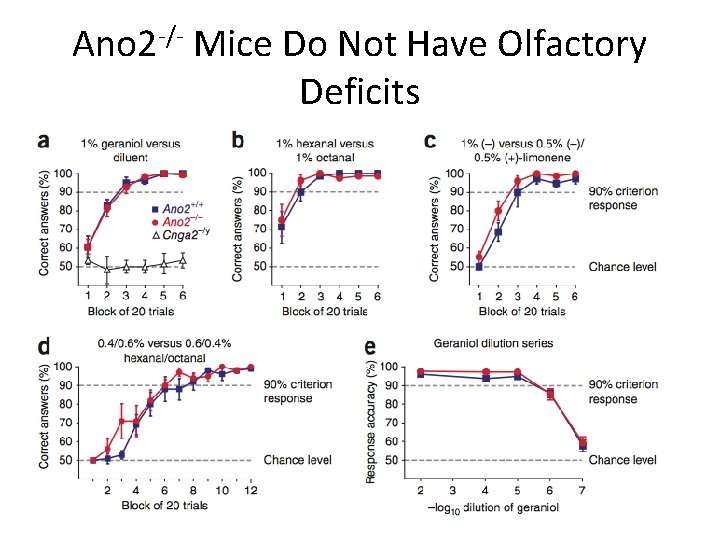
Ano 2 -/- Mice Do Not Have Olfactory Deficits

Conclusions • Ca 2+-activated Cl- currents are absent from MOE in Ano 2 -/- mice • Peak amplitude of olfactory epithelia responses decreased when stimulated with liquid in KO mice • No detectable difference in air phase EOG amplitude or in animal behavior tests • Ca 2+-activated Cl- channels not essential for olfaction

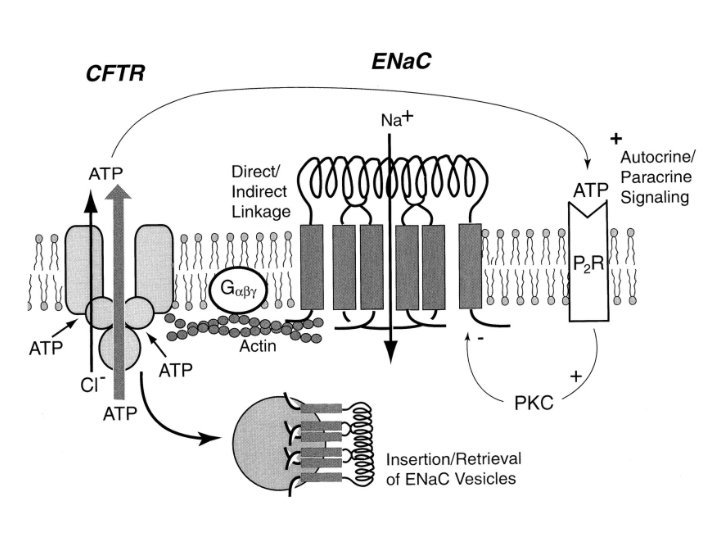
• A mutation of CFTR, a chloride channel crucial to maintaining salt and water homestasis in epethial tissues, is the cause of cystic fibrosis • CFTR is a ATP binding protien • ATP provides the energy required to open the pore of the channel • PKA phosphorylation regulates activity of channel

ATP Stimulates WT-CFTR
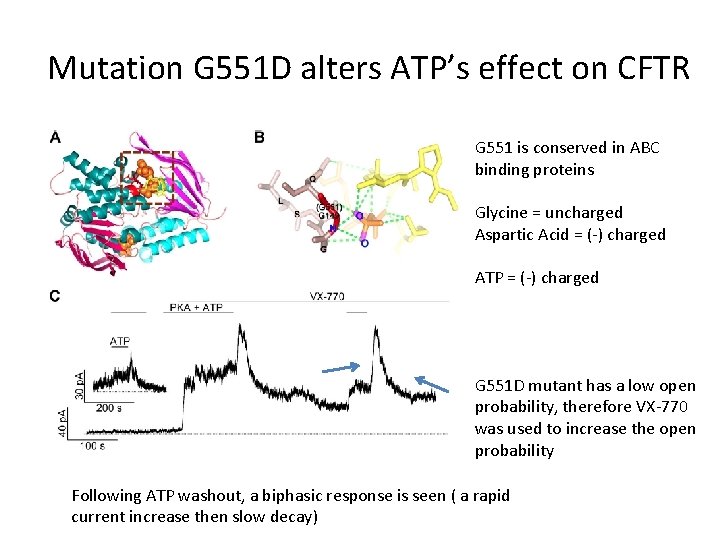
Mutation G 551 D alters ATP’s effect on CFTR G 551 is conserved in ABC binding proteins Glycine = uncharged Aspartic Acid = (-) charged ATP = (-) charged G 551 D mutant has a low open probability, therefore VX-770 was used to increase the open probability Following ATP washout, a biphasic response is seen ( a rapid current increase then slow decay)
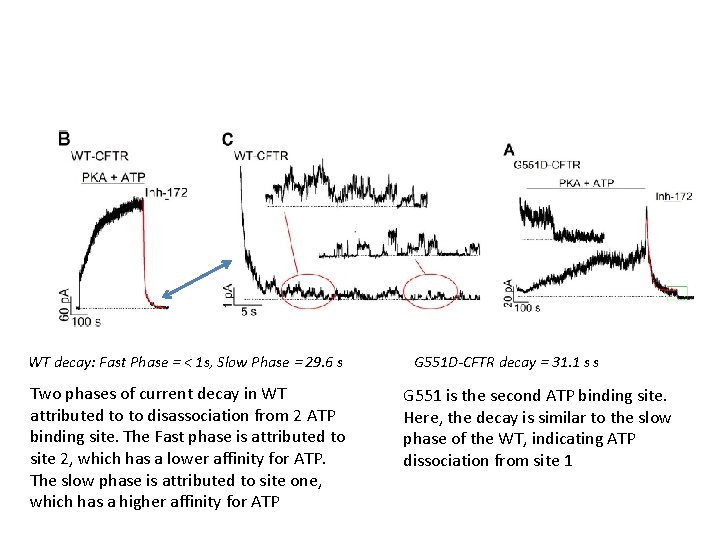
WT decay: Fast Phase = < 1 s, Slow Phase = 29. 6 s Two phases of current decay in WT attributed to to disassociation from 2 ATP binding site. The Fast phase is attributed to site 2, which has a lower affinity for ATP. The slow phase is attributed to site one, which has a higher affinity for ATP G 551 D-CFTR decay = 31. 1 s s G 551 is the second ATP binding site. Here, the decay is similar to the slow phase of the WT, indicating ATP dissociation from site 1
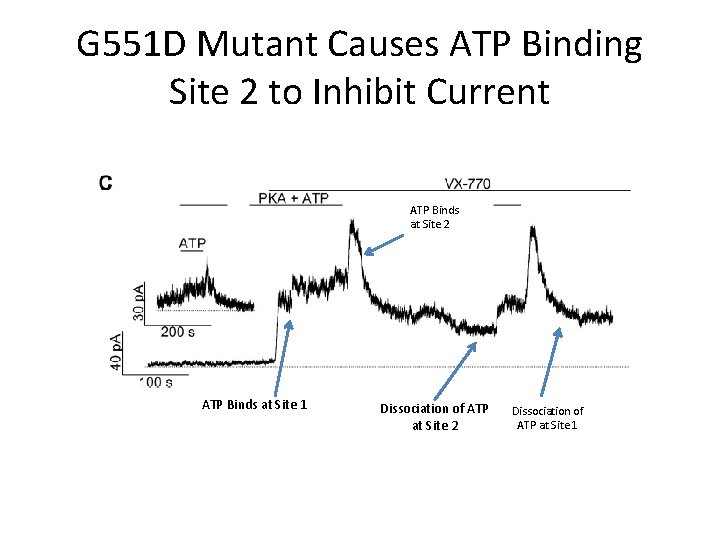
G 551 D Mutant Causes ATP Binding Site 2 to Inhibit Current ATP Binds at Site 2 ATP Binds at Site 1 Dissociation of ATP at Site 2 Dissociation of ATP at Site 1
![Reduction of [ATP] Increases Current in G 551 D Mutant ATP has a higher Reduction of [ATP] Increases Current in G 551 D Mutant ATP has a higher](http://slidetodoc.com/presentation_image_h/bc772f407b2e48e3326dac6b2225220d/image-55.jpg)
Reduction of [ATP] Increases Current in G 551 D Mutant ATP has a higher affinity for Site 1 Without VX-770, I is very low…. effect of change in [ATP] still apparent

Y 1219 in Site 2 Plays a Role in ATP Binding • To Test if G 551 D site 2 mutant was indeed inhibitory… • Mutation to other nonpolar/uncharged AA – Y 1219 F, Y 1219 I, Y 1219 G – Known to alter ATP affinity to Site 2
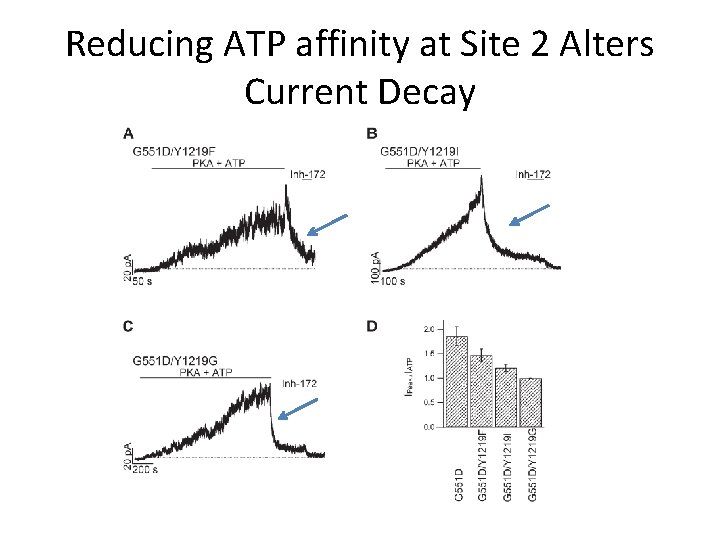
Reducing ATP affinity at Site 2 Alters Current Decay
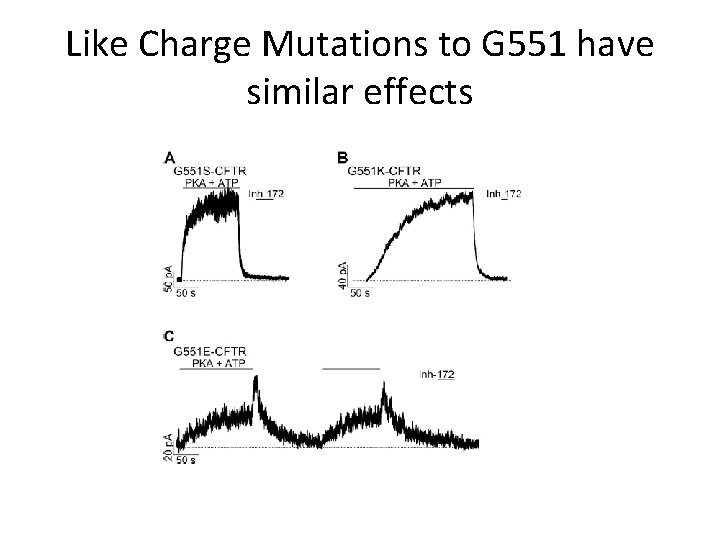
Like Charge Mutations to G 551 have similar effects
- Slides: 58