MEMANTINE AS AN ADJUNCT IN REFRACTORY SCHIZOPHRENIA OVERVIEW















- Slides: 37

MEMANTINE AS AN ADJUNCT IN REFRACTORY SCHIZOPHRENIA

OVERVIEW • Background: – Link between glutamatergic neurotransmission and schizophrenia • Overview of RCTs: – Lieberman et al. – Lucena et al. • Implication to practice

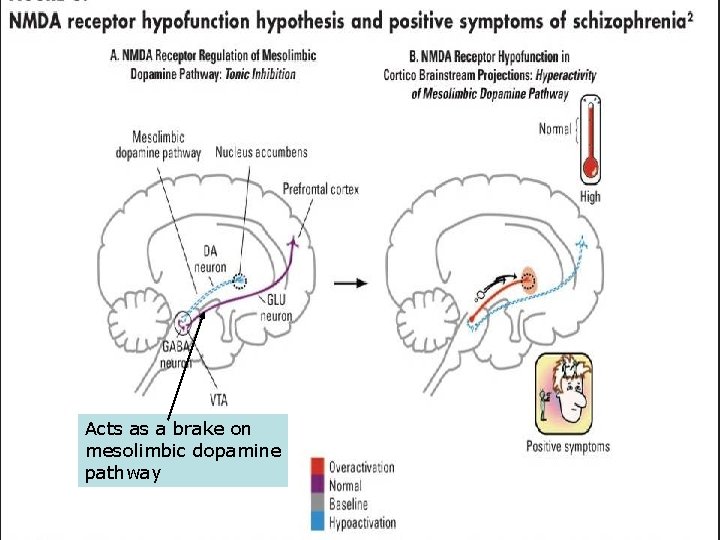
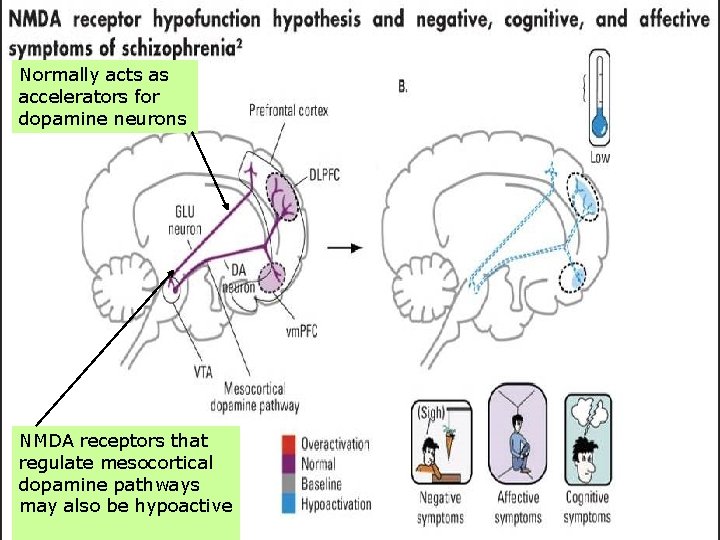
Background • Schizophrenia: Glutamate deregulation may be involved, mainly through Nmethyl-D-aspartate receptor (NMDAR) dysfunction • Memantine: Weak nonselective NMDAR antagonist

Acts as a brake on mesolimbic dopamine pathway

Normally acts as accelerators for dopamine neurons NMDA receptors that regulate mesocortical dopamine pathways may also be hypoactive

A Randomized , Placebo. Controlled Study of Memantine as Adjunctive Treatment in Patients with Schizophrenia

Purpose • To examine the efficacy and safety of memantine + atypical antipsychotics • Patients: Schizophrenic patients with partial response to antipsychotic treatment but with persistent residual psychopathology

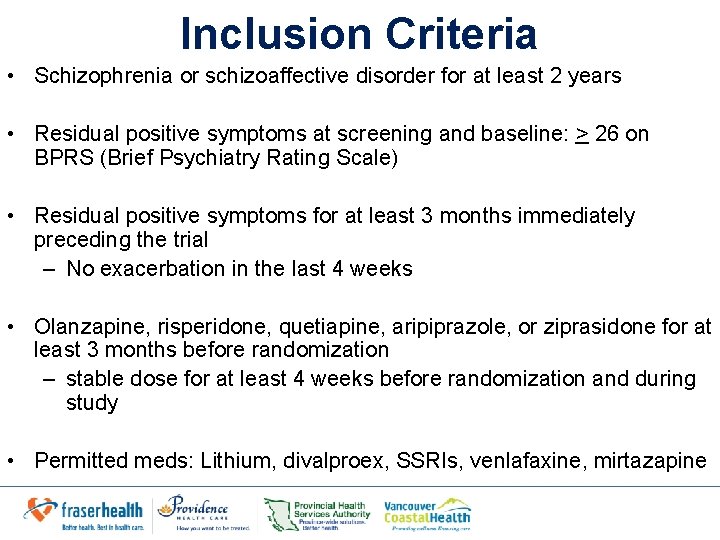
Inclusion Criteria • Schizophrenia or schizoaffective disorder for at least 2 years • Residual positive symptoms at screening and baseline: > 26 on BPRS (Brief Psychiatry Rating Scale) • Residual positive symptoms for at least 3 months immediately preceding the trial – No exacerbation in the last 4 weeks • Olanzapine, risperidone, quetiapine, aripiprazole, or ziprasidone for at least 3 months before randomization – stable dose for at least 4 weeks before randomization and during study • Permitted meds: Lithium, divalproex, SSRIs, venlafaxine, mirtazapine

Exclusion Criteria • A 20% change in total BPRS score from screening to baseline • Bipolar I disorder, either manic or mixed episode • Active suicide or homicide intent, or in the preceding 6 months • Organic brain disease, dementia, or a traumatic brain injury • Evidence or history of malignancy • Significant hematological, endocrine, cardiovascular, respiratory, renal, hepatic, or gastrointestinal disease

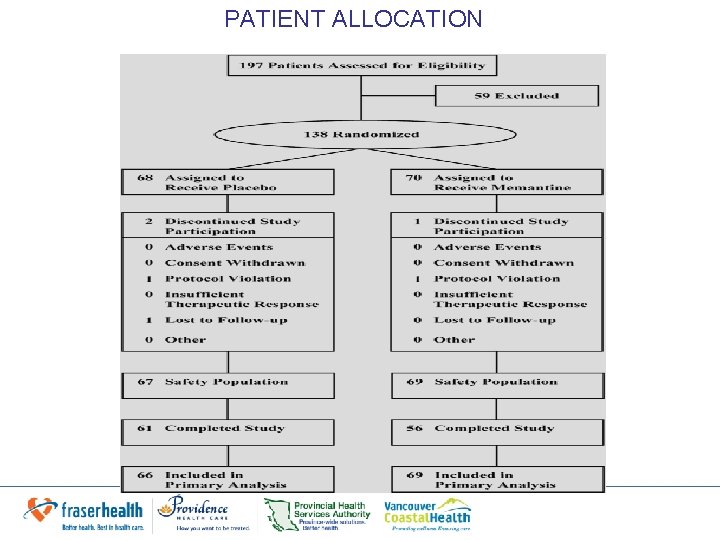
PATIENT ALLOCATION

Study Design • 8 -week, double-blind, placebo-controlled, randomized • Dosing titration: – Week 1: 5 mg/day – Week 2: 10 mg/day – Weeks 3 -8: 20 mg/day • Required sample size determination: – A clinically meaningful difference: 8. 5 points in total PANSS score • Intent-to-treat population • Primary efficacy parameter: change from baseline to week 8 in PANSS total score • Last Observation Carried Forward (LOCF) used for missing values

Safety Assessments • Adverse events and concomitant medications • Physical examination • Monitoring of EPS (Barnes Akathisia Scale, Abnormal Involuntary Movement Scale, Simpson-Angus Scale) • Vital sign measurements • ECG, laboratory tests, urine drug screen

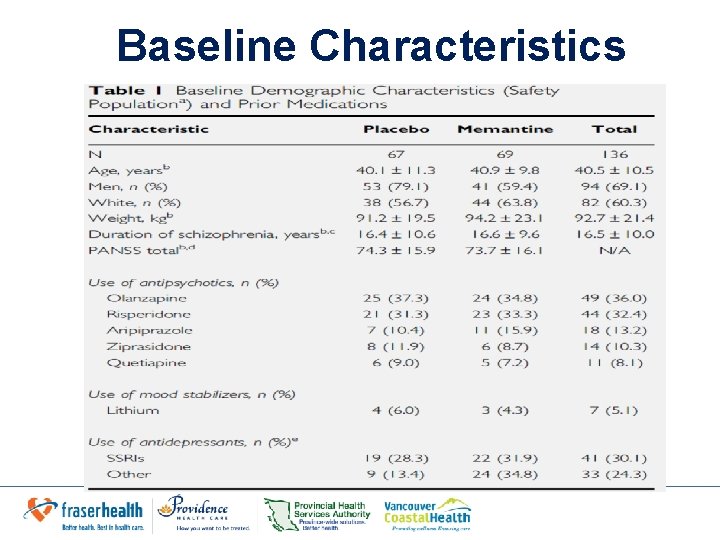
Baseline Characteristics

Outcome Measures • Primary outcome measure: – Total score on the positive and negative symptoms scale at baseline (week 0) and weeks 1, 2, 3, 4, 6 and 8 • Secondary outcome measures: – – – Positive and negative PANSS scores (all visits) PANSS responders ( >10% reduction in total PANSS score) Calgary Depression Scale for Schizophrenia (CDSS; week 0 and 8) Clinical Global Impressions of Severity (CGI-I; week 4, 6 and 8) Composite z-scores and total construct scores of Brief Assessment of Cognition in Schizophrenia (BACS; weeks 0, 4 and 8)

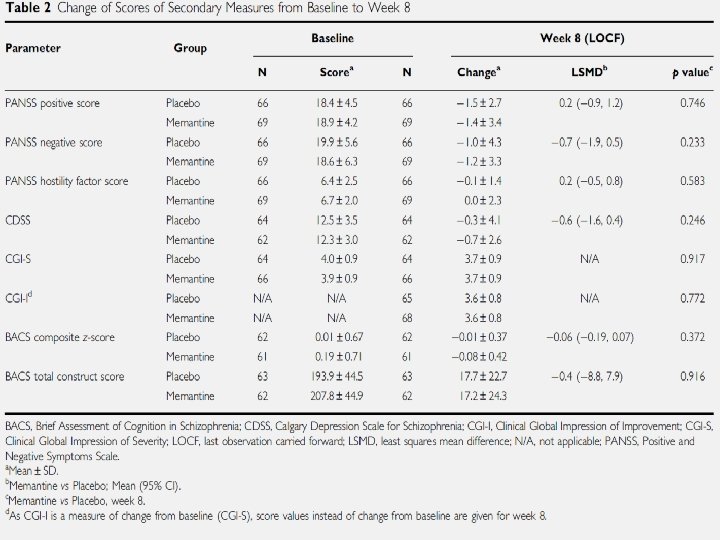
Results: Efficacy • Baseline: groups well matched except gender and use of non-SSRIs • Efficacy: – Mean change in total PANSS score: • -4. 5+ 10. 9 (Memantine) vs. -3. 7 +10. 2 – Secondary outcomes: Insignificant difference


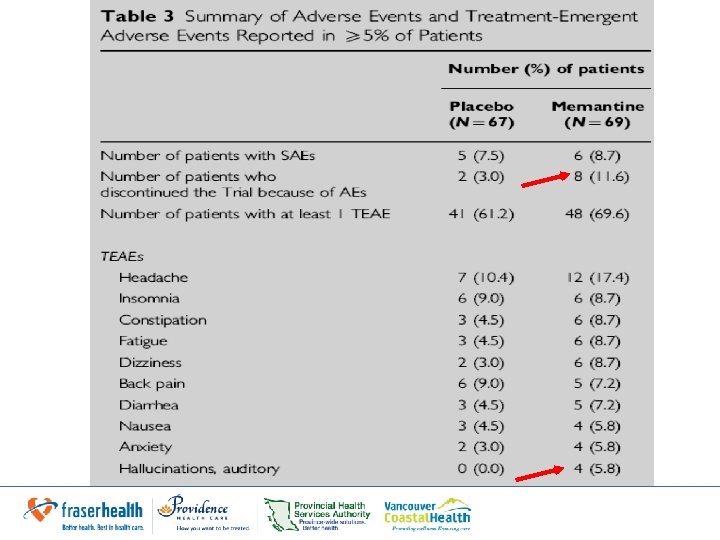
Results: Safety • Memantine: – Higher discontinuation rate – Most frequent SAE: exacerbation of schizophrenia (2. 9% vs. 6. 0% in placebo) – Dizziness & auditory hallucinations incidence 2 x that of placebo groups – Increases in auditory hallucinations not thought to be related to study medication by clinicians • Author still highlights this


Investigators’ Conclusions • No evidence of therapeutic benefits • Possibility of worsening of psychotic symptoms by memantine • Increased incidence of miscellaneous side effects • Participants may not have been optimal population – May be helpful in more pronounced cognitive impairment or with severe residual psychopathology • Safety profile less favourable than established profile

Factors affecting trial’s outcome • The variety of atypical antipsychotics used Not sufficiently powered to detect individual interactions with memantine • Relatively short duration of the trial • Relatively long titration period (3 weeks) exposure to a max daily dose for only 62. 5% time of the trial (5 weeks)

Improvement of Negative and Positive Symptoms in Treatment. Refractory Schizophrenia: A Double-Blind, Randomized Placebo -Controlled Trial With Memantine as Add-On Therapy to Clozapine

Study Design • Double Blind (Subject, Caregiver, Investigator, Outcome Assessor), placebo-controlled, randomized trial • Adult outpatients in Brazil with refractory schizophrenia • On clozapine over the last 10 years with partial remission of negative symptoms • Dosing titration: – Week 1: 5 mg/day – Week 2: 10 mg/day – Week 3: 15 mg/day – Week 4 -12: 20 mg/day

Methods • Assessments by trained psychiatrist blinded to treatment condition – Completed at weeks 4, 8 and 12 • Required sample size determined using assumption that: – A clinically meaningful difference b/w 2 groups = 10 points in total BPRS score

Exclusion Criteria • Any significant medical illness • Use of any additional psychotropic agent except benzodiazepines or substance abuse • Pregnant • At reproductive age and not taking contraceptives

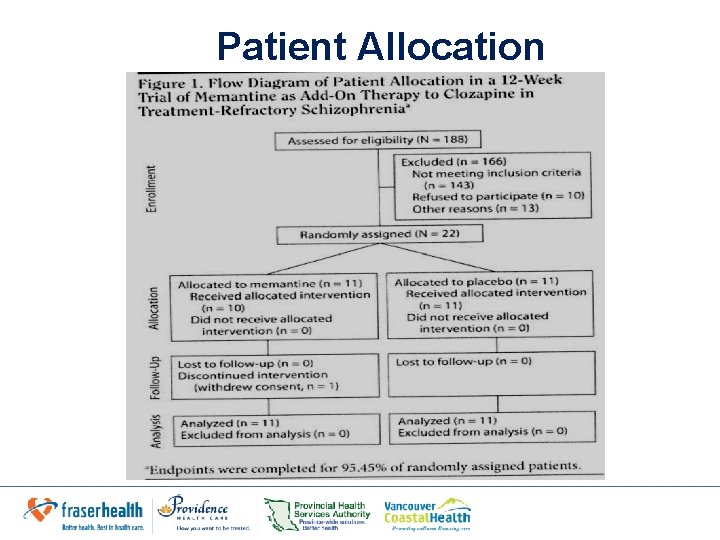
Patient Allocation

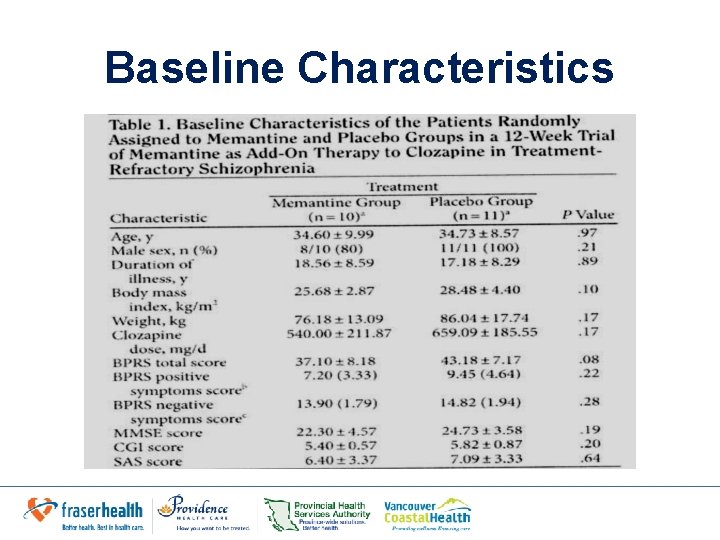
Baseline Characteristics

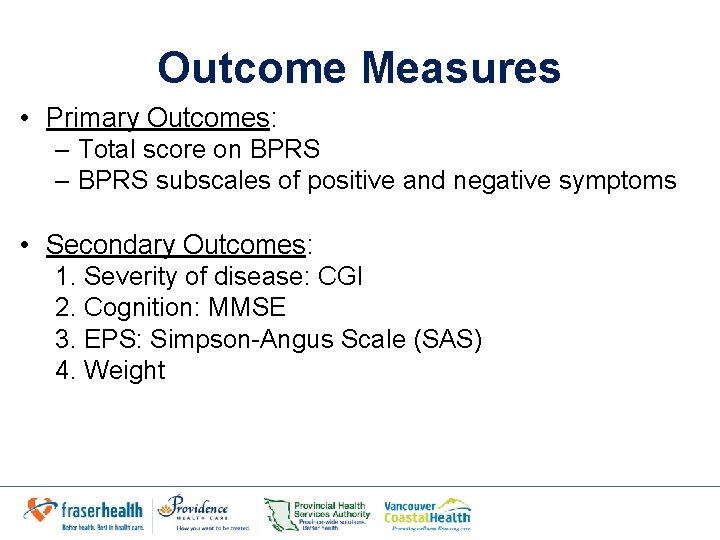
Outcome Measures • Primary Outcomes: – Total score on BPRS – BPRS subscales of positive and negative symptoms • Secondary Outcomes: 1. Severity of disease: CGI 2. Cognition: MMSE 3. EPS: Simpson-Angus Scale (SAS) 4. Weight

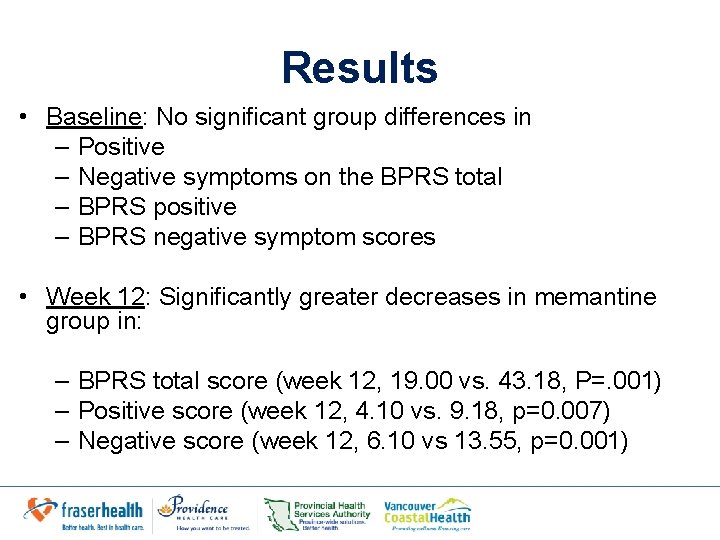
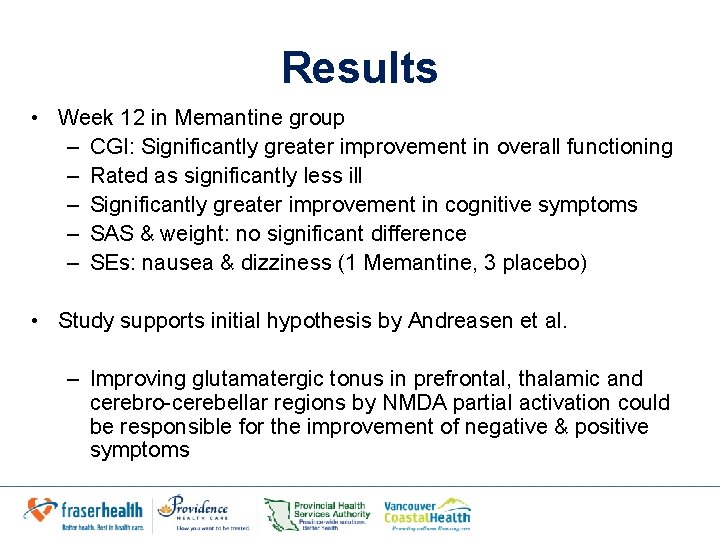
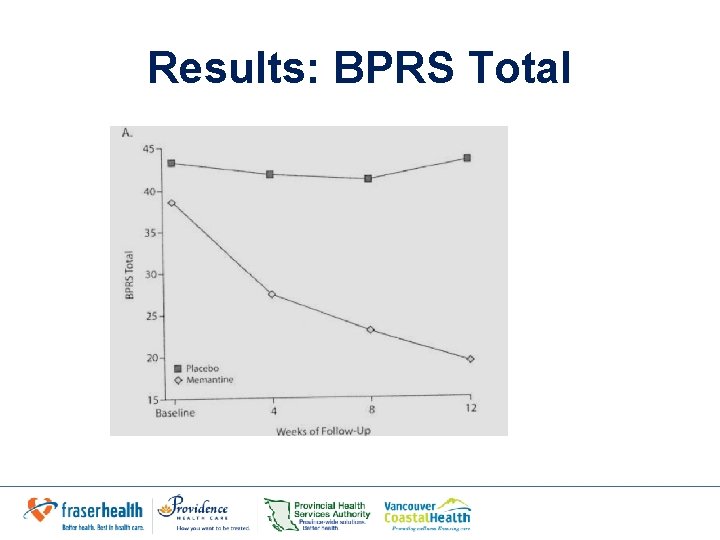
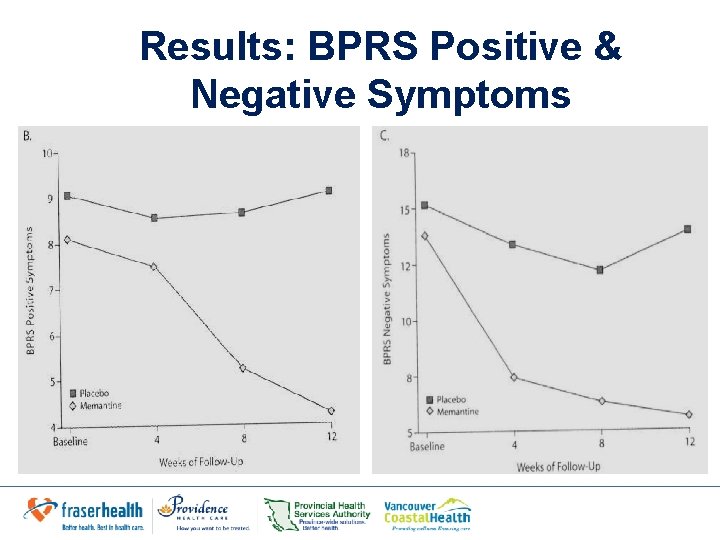
Results • Baseline: No significant group differences in – Positive – Negative symptoms on the BPRS total – BPRS positive – BPRS negative symptom scores • Week 12: Significantly greater decreases in memantine group in: – BPRS total score (week 12, 19. 00 vs. 43. 18, P=. 001) – Positive score (week 12, 4. 10 vs. 9. 18, p=0. 007) – Negative score (week 12, 6. 10 vs 13. 55, p=0. 001)

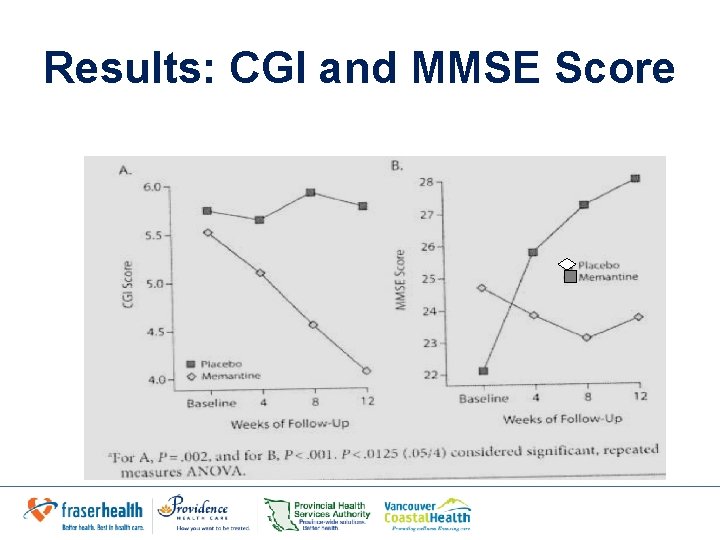
Results • Week 12 in Memantine group – CGI: Significantly greater improvement in overall functioning – Rated as significantly less ill – Significantly greater improvement in cognitive symptoms – SAS & weight: no significant difference – SEs: nausea & dizziness (1 Memantine, 3 placebo) • Study supports initial hypothesis by Andreasen et al. – Improving glutamatergic tonus in prefrontal, thalamic and cerebro-cerebellar regions by NMDA partial activation could be responsible for the improvement of negative & positive symptoms

Results: BPRS Total

Results: BPRS Positive & Negative Symptoms

Results: CGI and MMSE Score

Clozapine & Memantine • Clozapine increases expression of NMDARs and glutamate metabotropic receptors (m. Glu. Rs) • Hyper expression of m. Glu. R increased brain-derived neurotrophic factor (BDNF) • Chronic clozapine elevated m. Glu. R 5 improved glutametergic tonus • Special glutamatergic environment – Improvement with combination vs. failure with other associations – Appears to stabilize dopaminergic neurons dampening both hyperactivity and hypoactivity

Investigators’ Conclusion • Memantine improved positive symptoms without worsening of psychosis: broader actions than previously realized • Can prevent neuronal damage prevents dopamine deficit • Thus may act like antipsychotics by: – chronically reducing neuronal oxidative stress in treated patients – decrease neuroprogression & death • Trial supports the use of memantine as an adjunctive to clozapine

Limitations • Baseline: placebo group had slightly more severe symptoms on the BPRS and its subscales – Overestimation of memantine’s efficacy – Adjustment was done using ANCOVA – Less refractory more likely to respond • Serum clozapine levels not measured – Adherence? • Larger trials with longer follow-up period required • MMSE not the most sensitive measure of cognition

Implications to Practice • Could be tried in patients with partial response to clozapine • May help with negative and cognitive symptoms • RCTs with more subjects and longer trials required • Positive results cannot be generalized to patients with less severe symptoms and without clozapine use

References • Lieberman et al. A Randomized, Placebo-Controlled Study of Memantine as Adjunctive Treatment in Patients with Schizophrenia. Neuropsychopharmacology (2009) 34, 1322– 1329 • Lucena et al. Improvement of negative and positive symptoms in treatment-refractory schizophrenia: a double-blind, randomized, placebo-controlled trial with memantine as add-on therapy to clozapine. J Clin Psychiatry. 2011 Aug; 72(8): 1157. • Stahl Stephen M. Beyond the Dopamine Hypothesis to the NMDA Glutamate Receptor Hypofunction Hypothesis of Schizophrenia CNS Spectr. 2007; 12(4): 265 -268. Available from: http: //www. cnsspectrums. com/aspx/articledetail. aspx? articleid=1037