Melting and boiling point giant structures Substances with
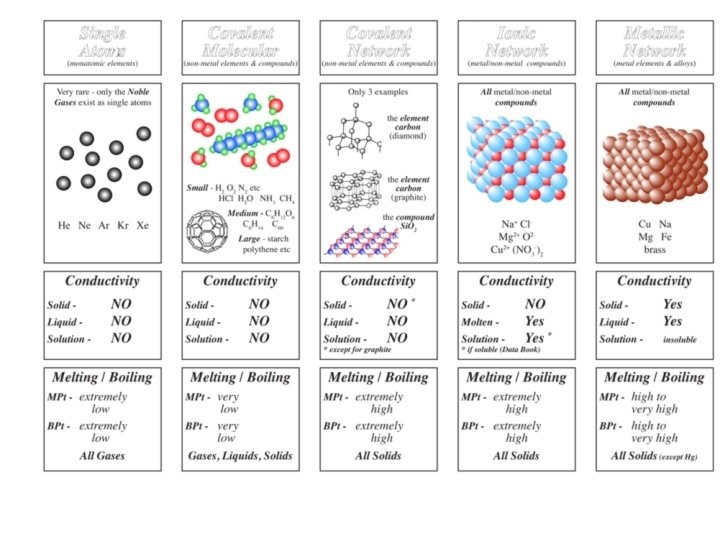
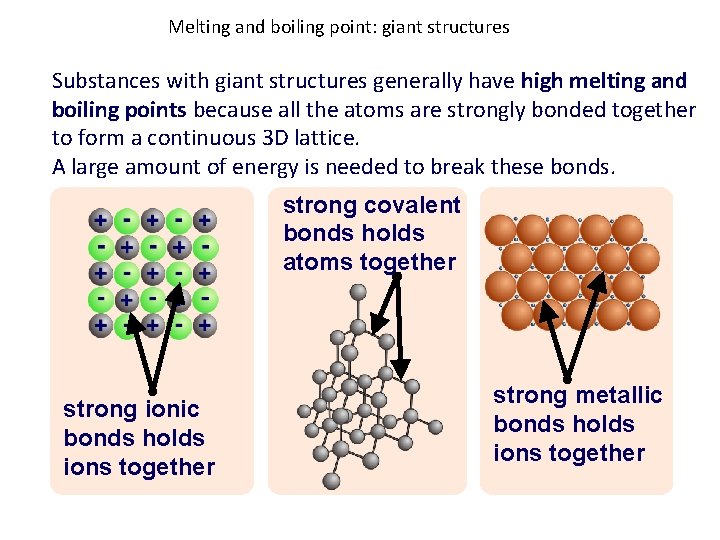
Melting and boiling point: giant structures Substances with giant structures generally have high melting and boiling points because all the atoms are strongly bonded together to form a continuous 3 D lattice. A large amount of energy is needed to break these bonds. strong covalent bonds holds atoms together strong ionic bonds holds ions together strong metallic bonds holds ions together
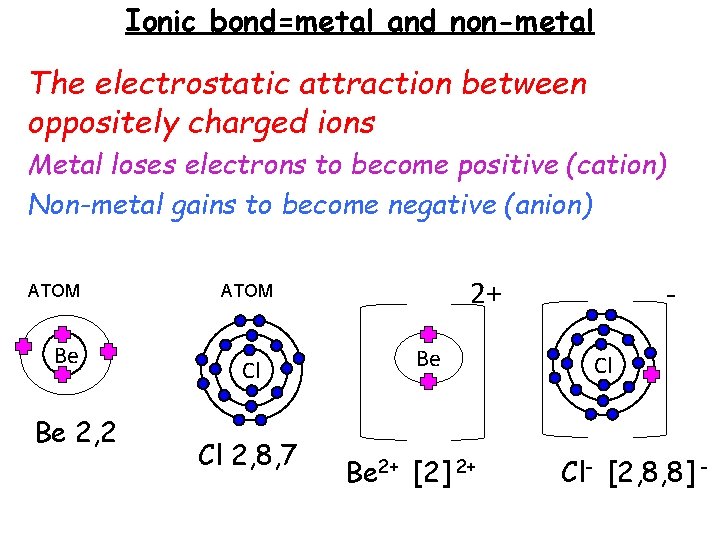
Ionic bond=metal and non-metal The electrostatic attraction between oppositely charged ions Metal loses electrons to become positive (cation) Non-metal gains to become negative (anion) ATOM Be Be 2, 2 2+ ATOM Cl Cl 2, 8, 7 Be Be 2+ [2] 2+ Cl Cl- [2, 8, 8] -

Ionic bonds ALWAYS FORM GIANT IONIC STRUCTURES not molecules High m. p. and b. p. - lots of strong ionic bonds in the giant ionic lattice, lots of energy to break. Conducts electricity when dissolved or molten- free moving ions, can conduct (solid= ions in fixed position cannot move) Soluble in water-water is polar so opposite charges attract ions out of the lattice.
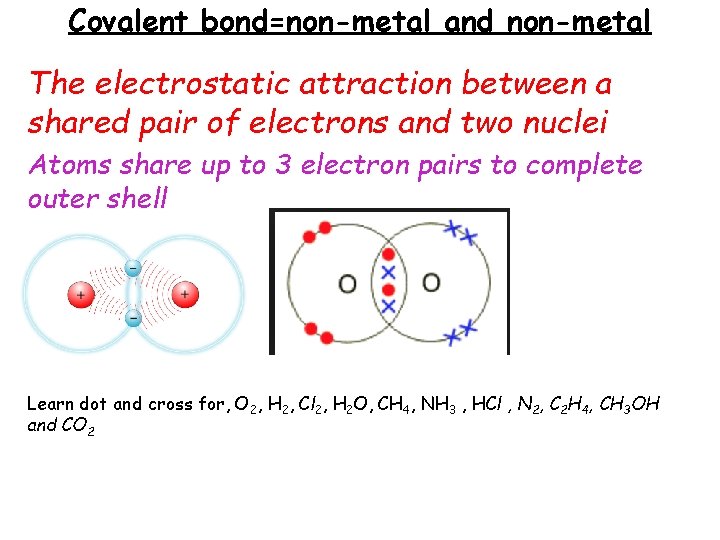
Covalent bond=non-metal and non-metal The electrostatic attraction between a shared pair of electrons and two nuclei Atoms share up to 3 electron pairs to complete outer shell Learn dot and cross for, O 2, H 2, Cl 2, H 2 O, CH 4, NH 3 , HCl , N 2, C 2 H 4, CH 3 OH and CO 2
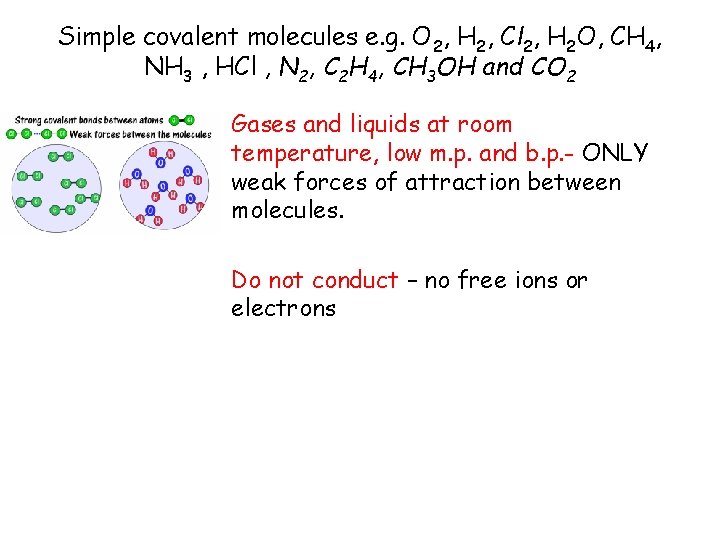
Simple covalent molecules e. g. O 2, H 2, Cl 2, H 2 O, CH 4, NH 3 , HCl , N 2, C 2 H 4, CH 3 OH and CO 2 Gases and liquids at room temperature, low m. p. and b. p. - ONLY weak forces of attraction between molecules. Do not conduct – no free ions or electrons
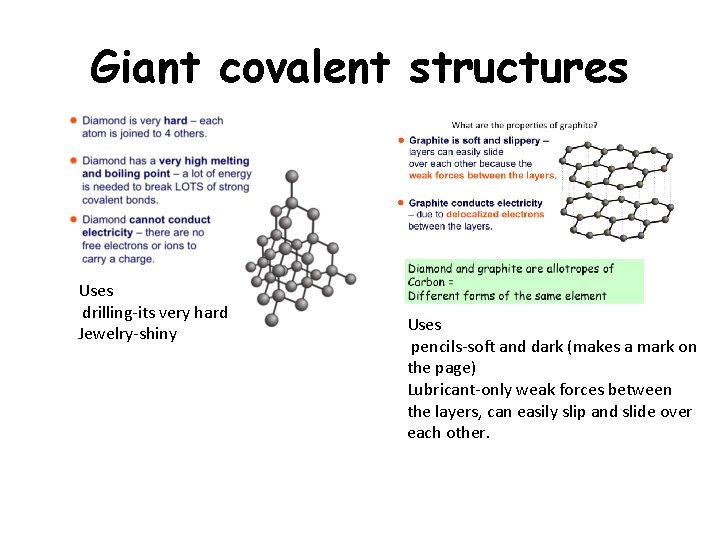
Giant covalent structures Uses drilling-its very hard Jewelry-shiny Uses pencils-soft and dark (makes a mark on the page) Lubricant-only weak forces between the layers, can easily slip and slide over each other.
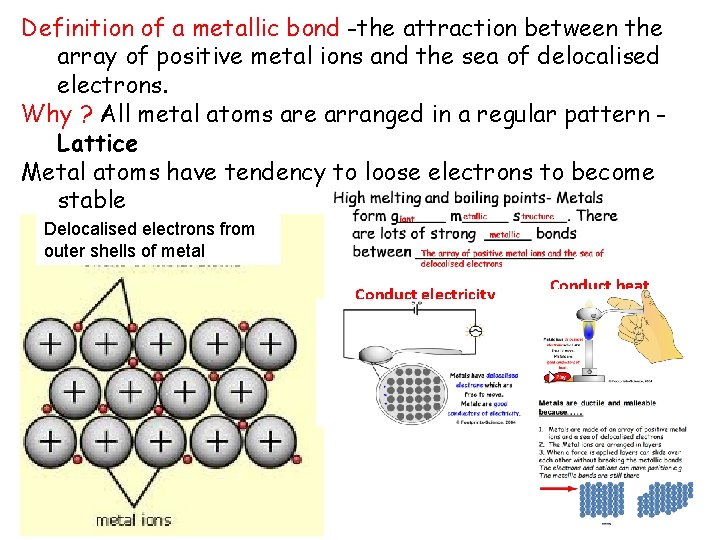
Definition of a metallic bond -the attraction between the array of positive metal ions and the sea of delocalised electrons. Why ? All metal atoms are arranged in a regular pattern Lattice Metal atoms have tendency to loose electrons to become stable Copy: Metal Structure Delocalised electrons from outer shells of metal Conduct electricity Conduct heat
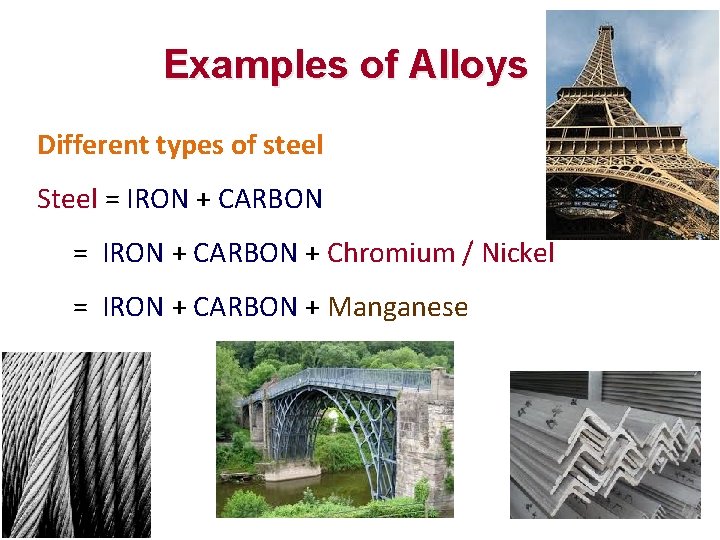
Examples of Alloys Different types of steel Steel = IRON + CARBON + Chromium / Nickel = IRON + CARBON + Manganese
Brass = Copper + Zinc. Bronze = Copper + Tin Solder = Zinc + Lead Amalgam = Mercury + Silver
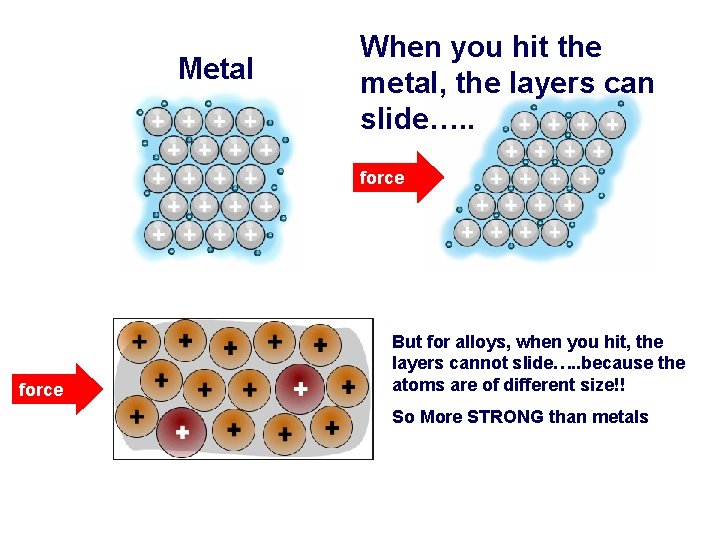
Metal When you hit the metal, the layers can slide…. . force But for alloys, when you hit, the layers cannot slide…. . because the atoms are of different size!! So More STRONG than metals
- Slides: 11