Mega Review in Chemistry Physical Setting Chemistry 1
































![2. Chemical reactions are either exothermic or endothermic [table I] 3. Chemical reactions must 2. Chemical reactions are either exothermic or endothermic [table I] 3. Chemical reactions must](https://slidetodoc.com/presentation_image_h/9d9a098f326f24858e645ae92d8fd24b/image-67.jpg)




































- Slides: 129

Mega Review in Chemistry

Physical Setting Chemistry 1. 2. 3. 4. 5. 6. Phases of Matter and Energy Gas Laws Atomic Structure Periodic Table Bonding Chemical Reactions

7. Solutions 8. Acids, Bases, and Salts 9. Electrochemistry 10. Kinetics and Equilibrium 11. Organic Chemistry 12. Nuclear Chemistry

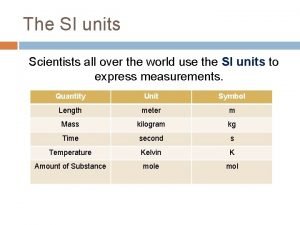
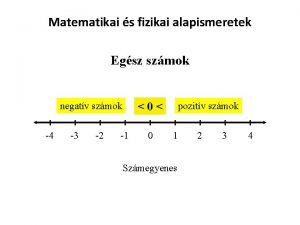
I. Matter and Energy Tables: B- Constants of Water H- Vapor Pressure T- Density and Heat and temperature

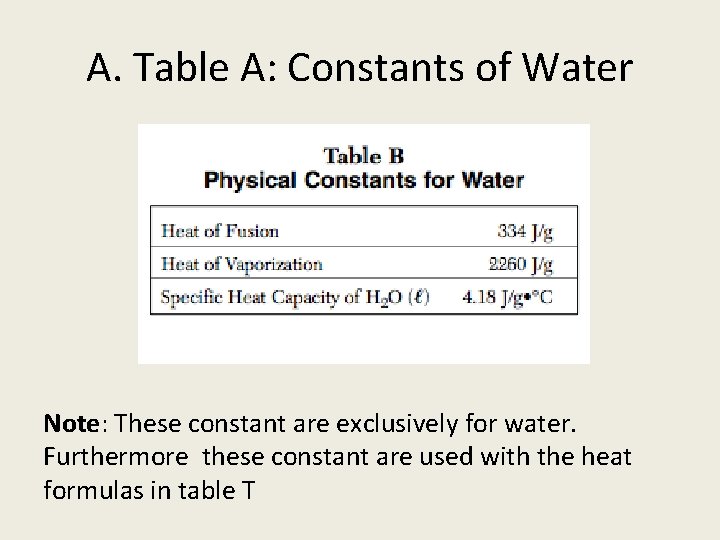
A. Table A: Constants of Water Note: These constant are exclusively for water. Furthermore these constant are used with the heat formulas in table T

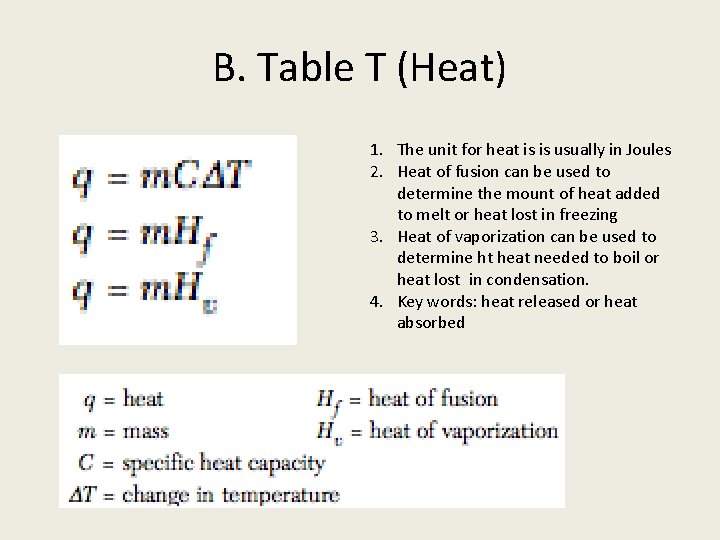
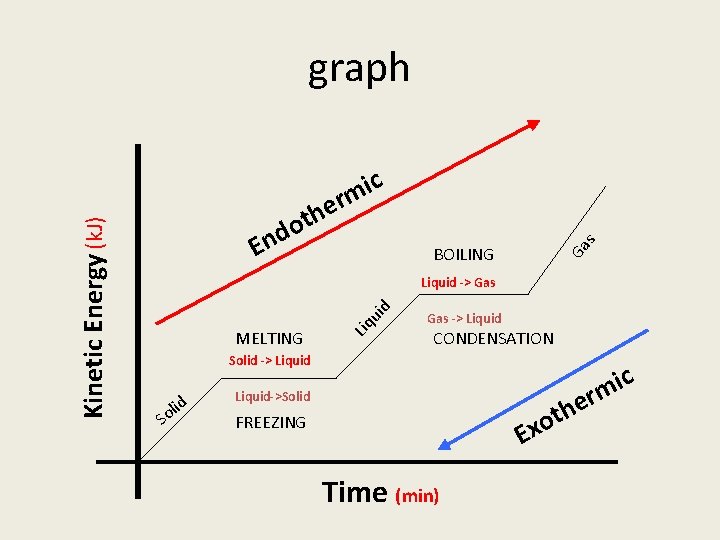
B. Table T (Heat) 1. The unit for heat is is usually in Joules 2. Heat of fusion can be used to determine the mount of heat added to melt or heat lost in freezing 3. Heat of vaporization can be used to determine ht heat needed to boil or heat lost in condensation. 4. Key words: heat released or heat absorbed

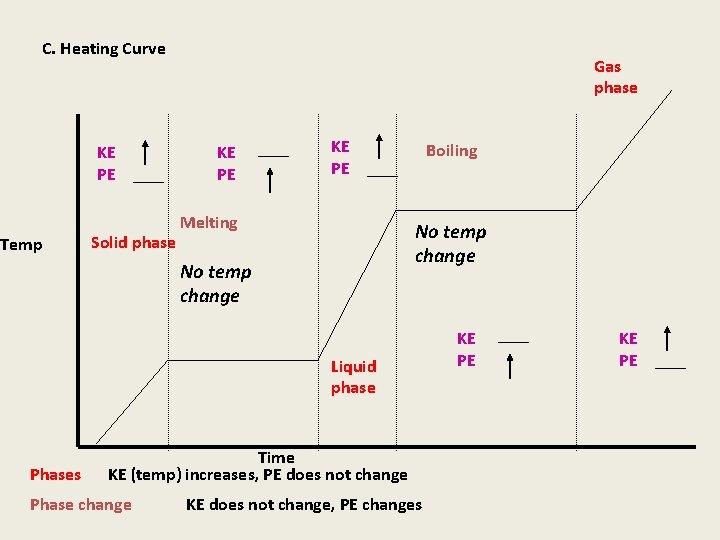
C. Heating Curve KE PE Temp Solid phase Gas phase KE PE Melting Boiling No temp change Liquid phase Phases Time KE (temp) increases, PE does not change Phase change KE does not change, PE changes KE PE

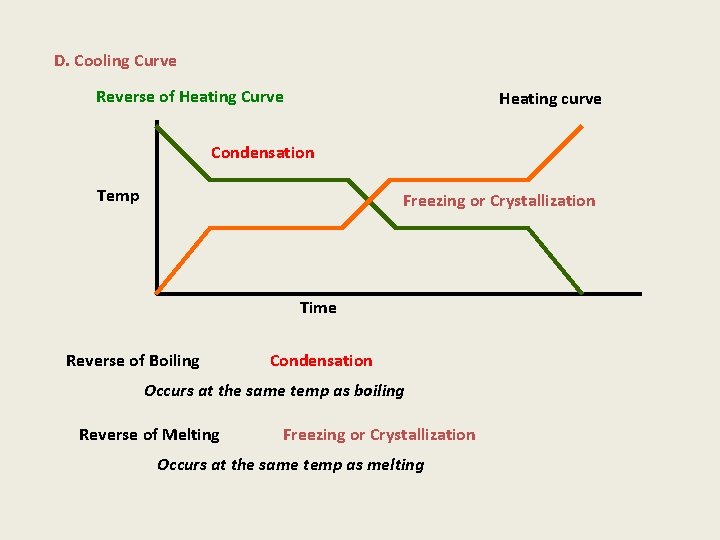
D. Cooling Curve Reverse of Heating Curve Heating curve Condensation Temp Freezing or Crystallization Time Reverse of Boiling Condensation Occurs at the same temp as boiling Reverse of Melting Freezing or Crystallization Occurs at the same temp as melting

Phase Changes Solid to liquid Melting Endothermic Liquid to gas Boiling Endothermic Gas to liquid Condensation Exothermic Liquid to solid Freezing Exothermic Solid to gas Sublimation Endothermic Gas to solid deposition Exothermic

s r e h t o BOILING Ga d n E c i m ui d Liquid -> Gas MELTING Liq Kinetic Energy (k. J) graph Gas -> Liquid CONDENSATION Solid -> Liquid S d oli r e h t o Liquid->Solid Ex FREEZING Time (min) c i m

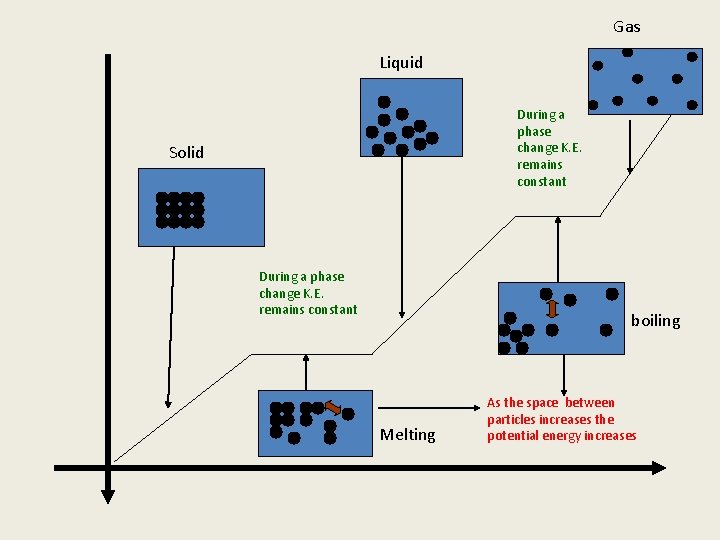
Gas Liquid During a phase change K. E. remains constant Solid During a phase change K. E. remains constant boiling Melting As the space between particles increases the potential energy increases

Key points thus far 1. The kinetic energy changes in the presence of one phase only: Solid, Liquid, and Gas 2. The kinetic energy remains CONSTANT during phase change (two phases present simultaneously) melting, boiling, condensation, and freezing. 3. The only thing that changes during a phase change is the potential energy

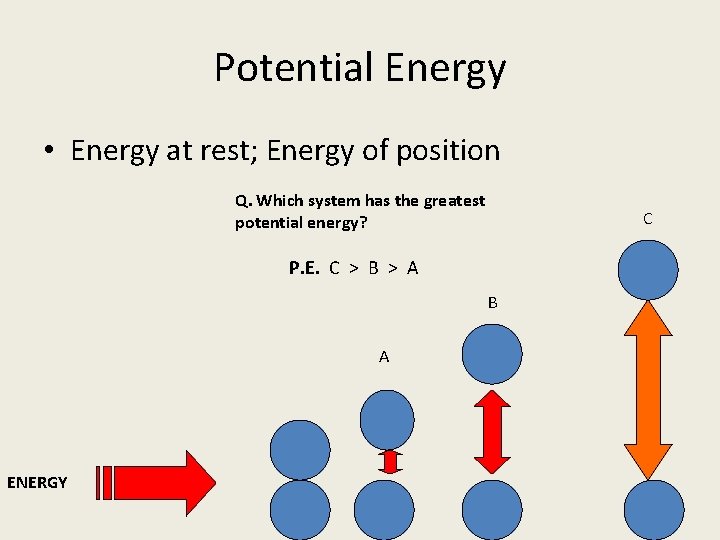
Potential Energy • Energy at rest; Energy of position Q. Which system has the greatest potential energy? C P. E. C > B > A B A ENERGY

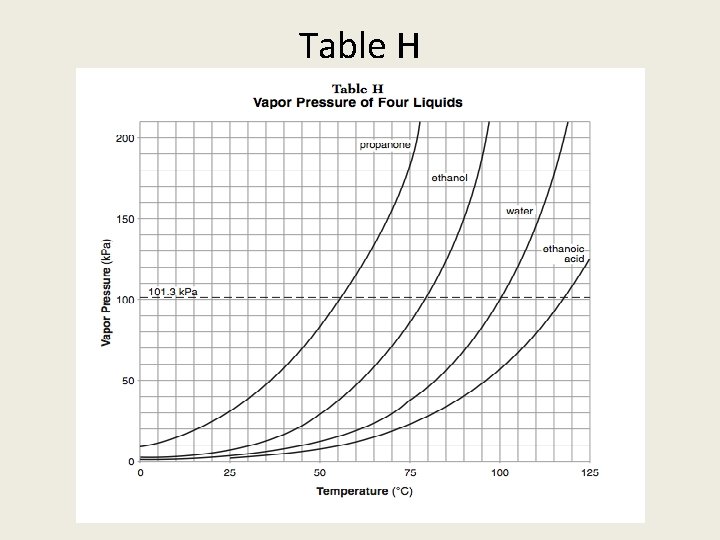
Table H Vapor pressure of a liquid = Atmospheric Pressure What occurs? BOILING

Table H






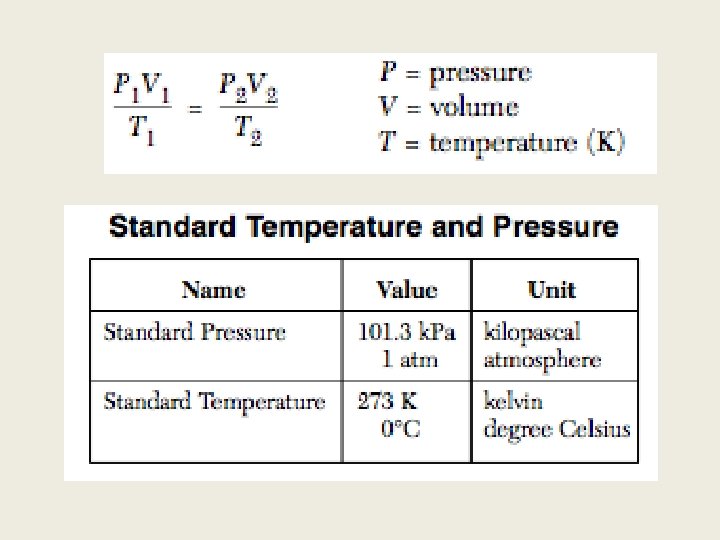
Unit 2: Gas Laws Table A: Standard Temperature and Pressure Table T: Combine Gas Formula


Gas Laws Temp constant P 1 V 1 =P 2 V 2 V P Boyle’s Law Combine gas formula Pressure Constant P 1 V 1/T 1 =P 2 V 2/T 2 V 1/T 1=V 2/T 2 V Note: Temp is always in Kelvin T Charles’ Law Volume constant The name is not specifically asked On the regents (Gay-Lussac’s only. P 1/T 1= P 2/T 2 p/t =p/t T P Gay-Lussac’s Law

Characteristics of an ideal 1 The molecules in a gas occupy no volume (that is, they are points). That is, gas particles have negligible volume thus gases occupy “empty space” 2. A gas consists of a collection of small particles traveling in straight-line, constant, random motion 3. The avg. kinetic energy of the ideal gas molecules is proportional to the absolute temp of the molecules 4. When molecules collide with each other, the collisions are elastic 5. The molecules of an ideal gas do not exert any attractive or repulsive forces on each other

Unit 3 Atomic Structure

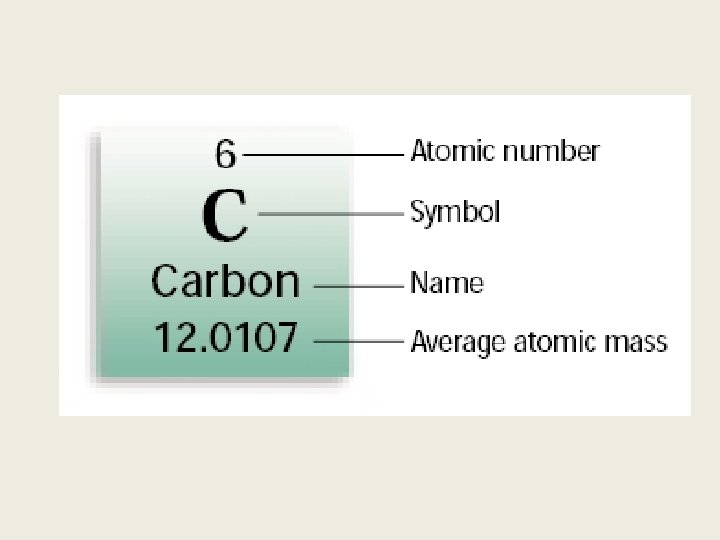
What you should know? 1. History of the atom 1. Determine the number of Protons, Electrons, and Neutrons 1. Use the Periodic table along with table S to explore chemical as well as physical properties of elements.

I. Theory of the Atom A. Ancient Greece (2000+ years ago) Democratus Believed that matter could not be continuously divided Matter consists of small indivisible particles “Atom” = indestructible Particle are in continuous motion Four elements make up all matter and energy Earth, Wind, Water, Fire, No scientific evidence to show this.

B. Dalton’s Atomic Theory - 1808 John Dalton proposed theory that: 1. All matter is composed of small particles which cannot be broken down (atoms) 2. All atoms of the same element are identical in size, mass and properties. Atoms of different elements are different in size, mass and properties 3. Atoms of different element combine in simple ratios to make compounds H 2 S Pb. O 2 4. In chemical reactions, atoms are combined, separated, or rearranged (No atoms are created or destroyed) So at this point we believe that an atom is like a small solid ball of matter that cannot be split up

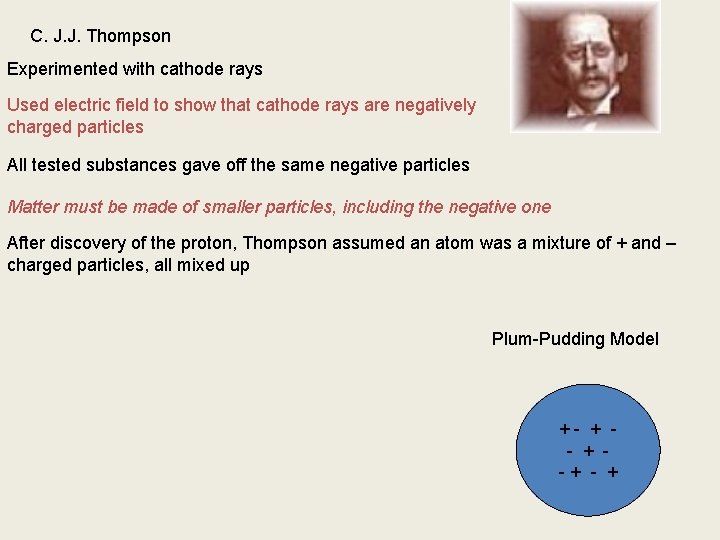
C. J. J. Thompson Experimented with cathode rays Used electric field to show that cathode rays are negatively charged particles All tested substances gave off the same negative particles Matter must be made of smaller particles, including the negative one After discovery of the proton, Thompson assumed an atom was a mixture of + and – charged particles, all mixed up Plum-Pudding Model +- + -+ - +

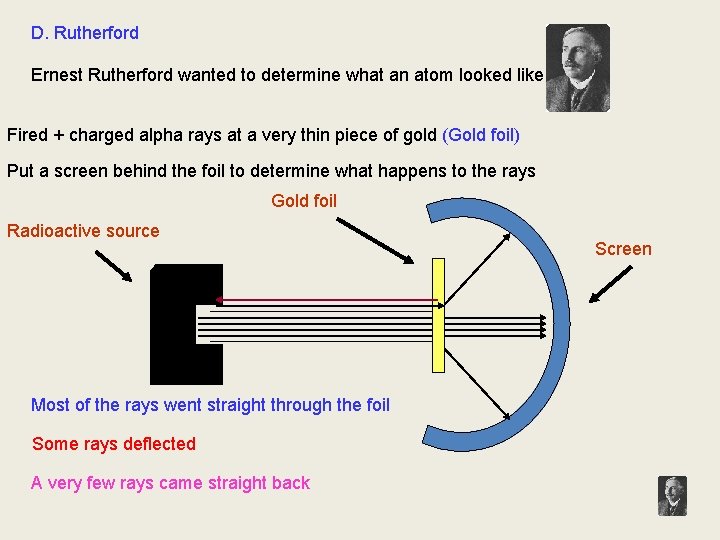
D. Rutherford Ernest Rutherford wanted to determine what an atom looked like. Fired + charged alpha rays at a very thin piece of gold (Gold foil) Put a screen behind the foil to determine what happens to the rays Gold foil Radioactive source Most of the rays went straight through the foil Some rays deflected A very few rays came straight back Screen

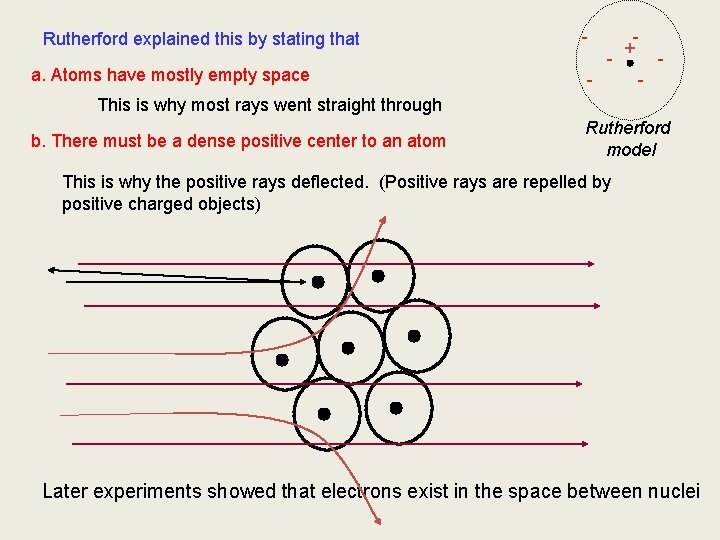
Rutherford explained this by stating that a. Atoms have mostly empty space - + - - This is why most rays went straight through b. There must be a dense positive center to an atom Rutherford model This is why the positive rays deflected. (Positive rays are repelled by positive charged objects) Later experiments showed that electrons exist in the space between nuclei

E. Bohr Neils Bohr looked at the arrangement of electrons Electrons exist in definite areas around the nucleus Energy levels Further from the nucleus, an electron had more energy M 3 e- L 2 K 1 ee- e- Electrons can gain energy and “jump” to higher levels nucleus They can then give off the energy as they jump back down Bohr named the levels K, L, M, N, O… with K begin closest to the nucleus Now renamed 1, 2, 3, 4… with 1 being closest to the nucleus and having the least amount of energy All atoms have the same types of energy levels - - - +- Planetary model

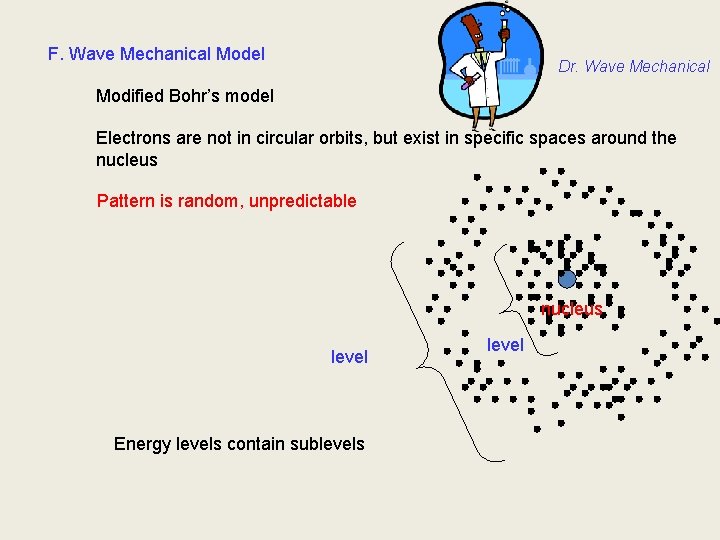
F. Wave Mechanical Model Dr. Wave Mechanical Modified Bohr’s model Electrons are not in circular orbits, but exist in specific spaces around the nucleus Pattern is random, unpredictable nucleus level Energy levels contain sublevels level

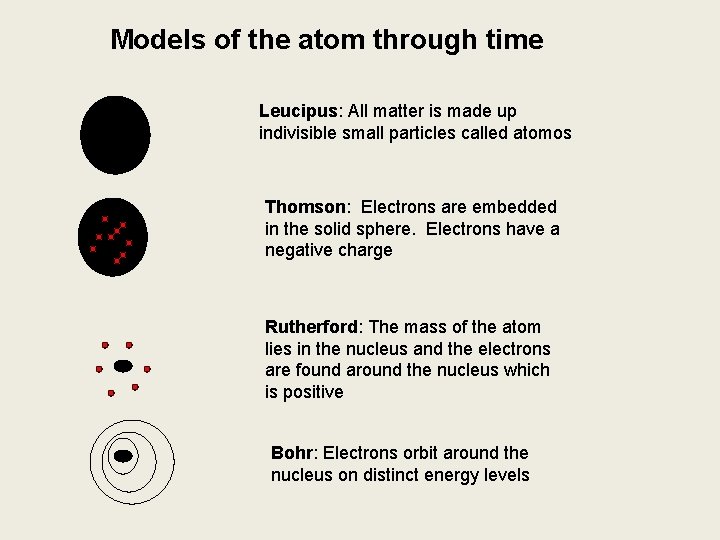
Models of the atom through time Leucipus: All matter is made up indivisible small particles called atomos Thomson: Electrons are embedded in the solid sphere. Electrons have a negative charge Rutherford: The mass of the atom lies in the nucleus and the electrons are found around the nucleus which is positive Bohr: Electrons orbit around the nucleus on distinct energy levels

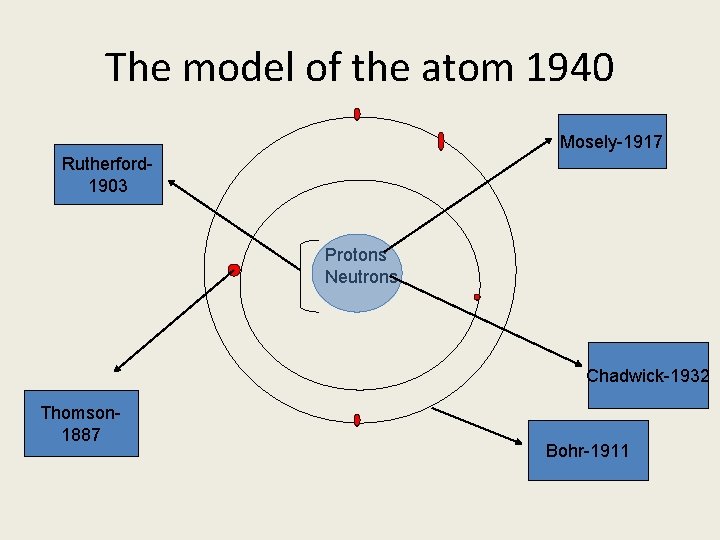
The model of the atom 1940 Mosely-1917 Rutherford 1903 Protons Neutrons Chadwick-1932 Thomson 1887 Bohr-1911

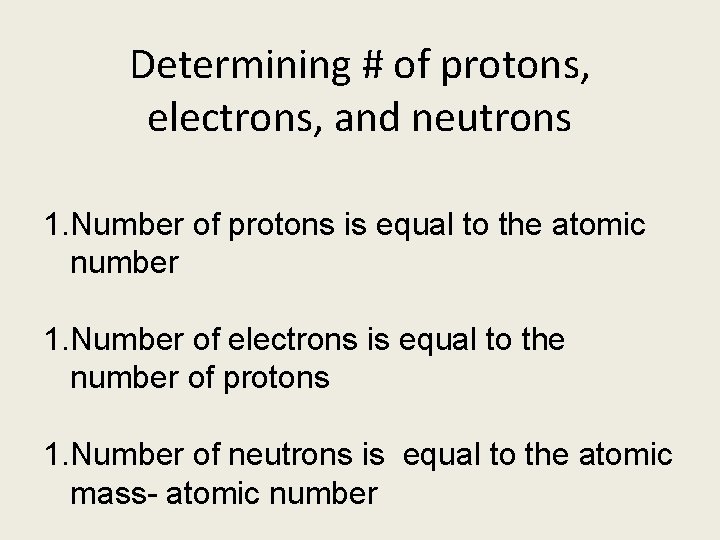
Determining # of protons, electrons, and neutrons 1. Number of protons is equal to the atomic number 1. Number of electrons is equal to the number of protons 1. Number of neutrons is equal to the atomic mass- atomic number


Unit 4 Periodic Table

What should I know? 1. The main organization of the table: metals, semi-metals, and nonmetals 2. The basic trends within the periodic table: a. Electronegativity b. Ionization energy c. Atomic radius 3. Groups Group #1 - alkali Group #2 - alkaline Group #3 -#12 - Transition elements Group #17 - Halogens Group #18 - noble gasas 4. Periods:


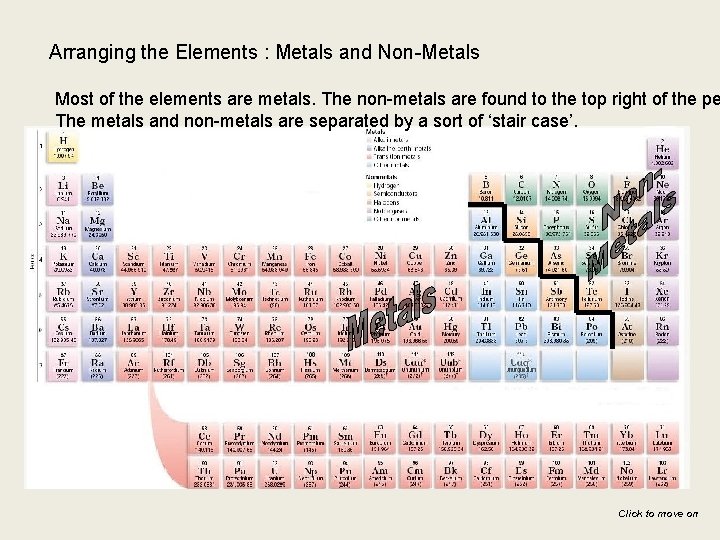
Arranging the Elements : Metals and Non-Metals Most of the elements are metals. The non-metals are found to the top right of the pe The metals and non-metals are separated by a sort of ‘stair case’. Click to move on

Semi-metals Metalloids Elements that share metallic and nonmetallic properties B , Si, Ge, As, Sb, Te

Diatomic elements that exist as paired atoms in their elemental state: H 2 F 2 Cl 2 Br 2 I 2 O 2 N 2 “ Brinclhof”

Properties of Metals have the following properties : §They conduct electrical energy well §They conduct thermal energy well §They are shiny §They are malleable (can be hammered into shape) §They are ductile (can be drawn out into wires) §They are sonorous (make a ringing sound when hit) §All except mercury are solids at room temperature Click to move on

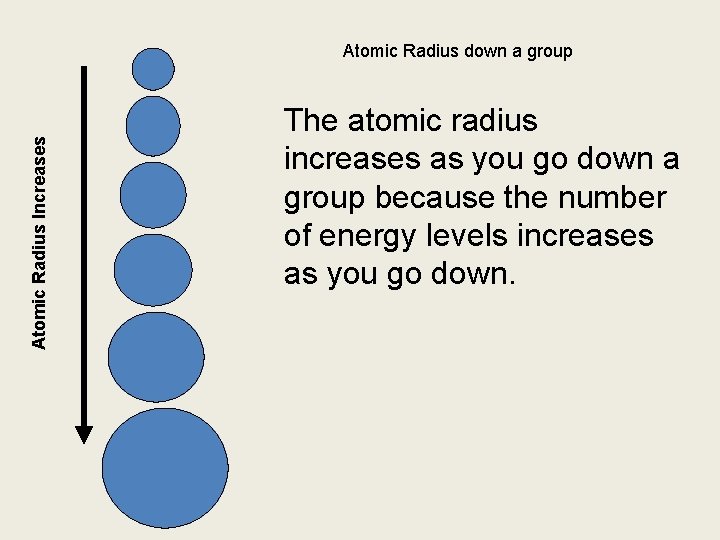
Atomic Radius Increases Atomic Radius down a group The atomic radius increases as you go down a group because the number of energy levels increases as you go down.

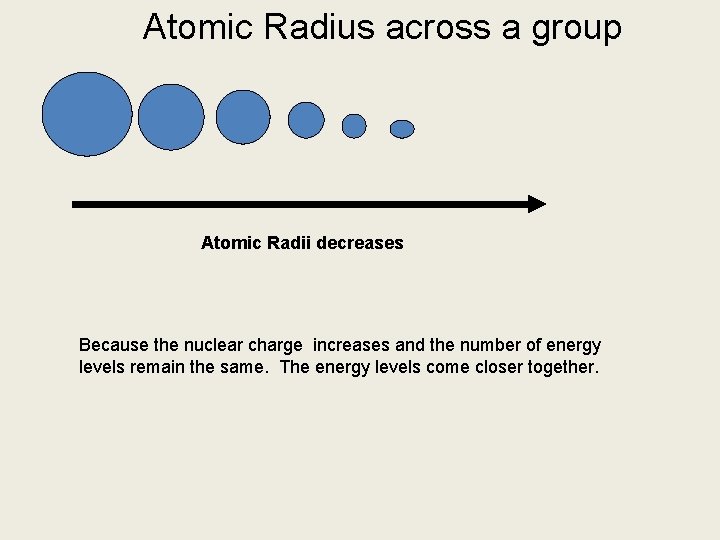
Atomic Radius across a group Atomic Radii decreases Because the nuclear charge increases and the number of energy levels remain the same. The energy levels come closer together.

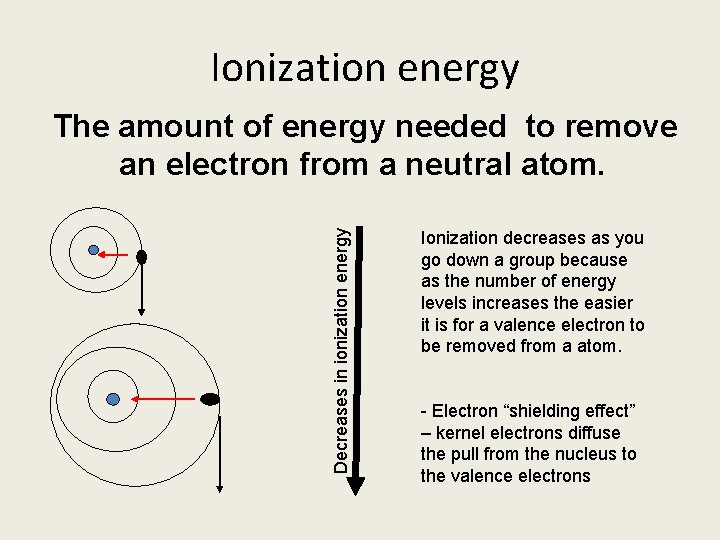
Ionization energy Decreases in ionization energy The amount of energy needed to remove an electron from a neutral atom. Ionization decreases as you go down a group because as the number of energy levels increases the easier it is for a valence electron to be removed from a atom. - Electron “shielding effect” – kernel electrons diffuse the pull from the nucleus to the valence electrons

Electronegativity Pull for electrons between two atoms that are bonded covalently Electronegativity and ionization energy share the same trend within the periodic table. That is, they both increase as you go across the period and decrease as you go down a group. Metals tend to have low ionization and low electronegativities while nonmetal tend to have high ionization and high Electronegativity

Unit 5 Bonding

What should you know? 1. There are two three types of bonding: ionic, covalent, and metallic 2. Through bonding compounds are formed: ionic compounds and covalent (molecular ) compounds. 3. Valence electrons play a key role in bonding. 4. Through bonding atoms want to obtain a “noble” gas electron configuration. That is, each an octet configuration. 5. When bonds are broken energy is absorbed and when formed energy is released. 6. Lewis structure depict the interaction of valence electrons within a bond. 7. There are polar and nonpolar molecules as well as polar and nonpolar covalent bonds.

Compounds vs Mixtures 1. Compounds have a fix ratio. If the ratio changes the compound changes chemically and physically. 1. Mixtures can vary and they continue being chemically and physically the same. Salt water is salty no matter how much salt you use to make a salt mixture 1. Compounds CANNOT be separated by physical means 1. Mixtures can be separated by physical means: distillation, decantation, filtration, etc.

I. Possible types of bonds • Non-metal + Metal Ionic bonds • Non-metal + Non-metal Covalent bonds • Metal + Metallic

Ionic Bonding

Covalent Bonding

H Potential Energy H H H Free atoms H Energy Bonded atoms H 2 H H H H 2 Formation of Chemical Bonds results in the release of energy Breaking chemical bonds requires the addition of energy Making Bonds Chemical Energy Exothermic Breaking Bonds Energy released as a bond is formed Energy used to break chemical bonds Chemical energy is considered potential (stored) energy. Endothermic

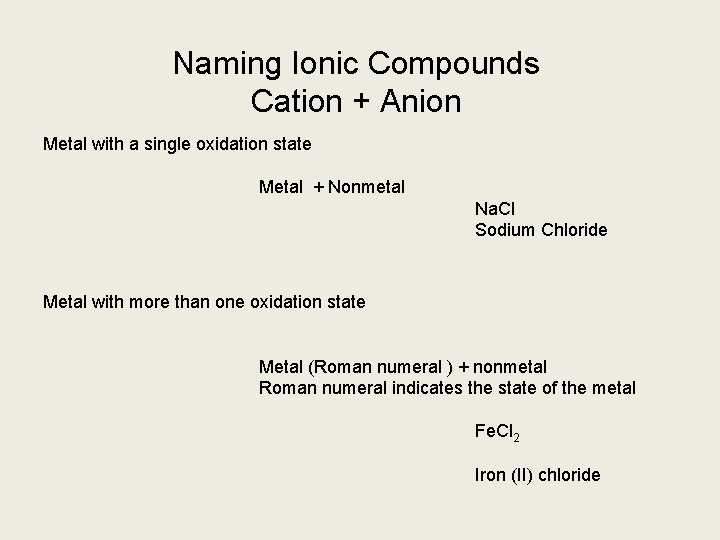
Naming Ionic Compounds Cation + Anion Metal with a single oxidation state Metal + Nonmetal Na. Cl Sodium Chloride Metal with more than one oxidation state Metal (Roman numeral ) + nonmetal Roman numeral indicates the state of the metal Fe. Cl 2 Iron (II) chloride

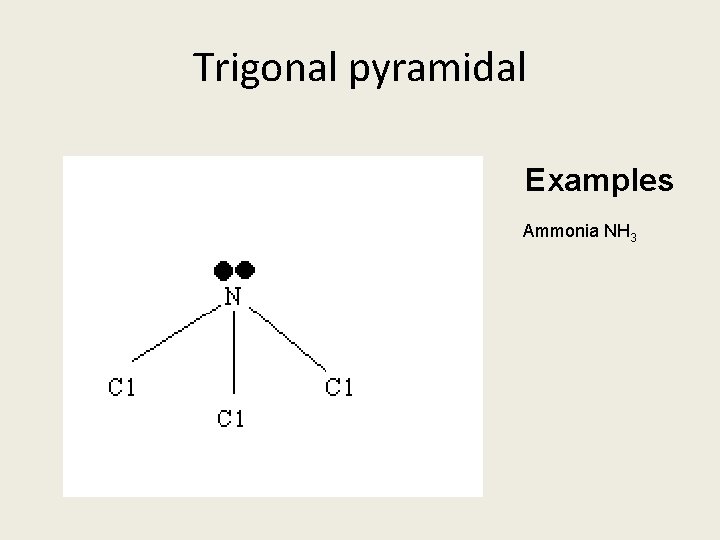
Covalent Compounds form molecules Four molecule shapes you need to know: 1. Linea 2. Bent 3. Tetrahedral 4. Pyramidal

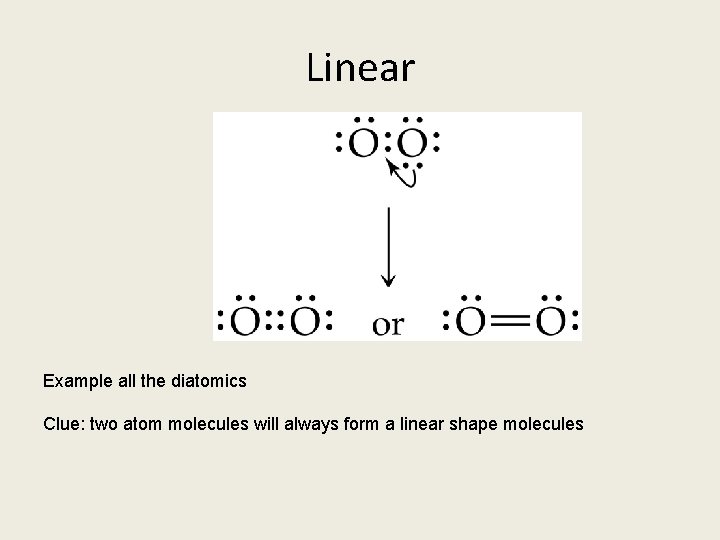
Linear Example all the diatomics Clue: two atom molecules will always form a linear shape molecules

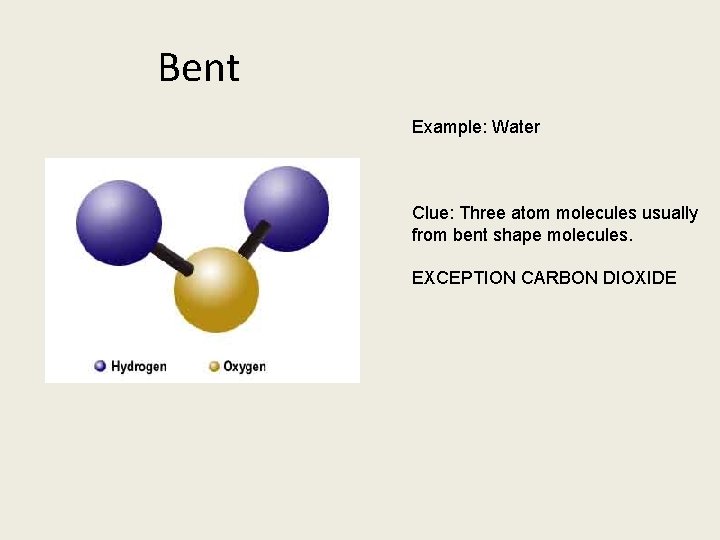
Bent Example: Water Clue: Three atom molecules usually from bent shape molecules. EXCEPTION CARBON DIOXIDE

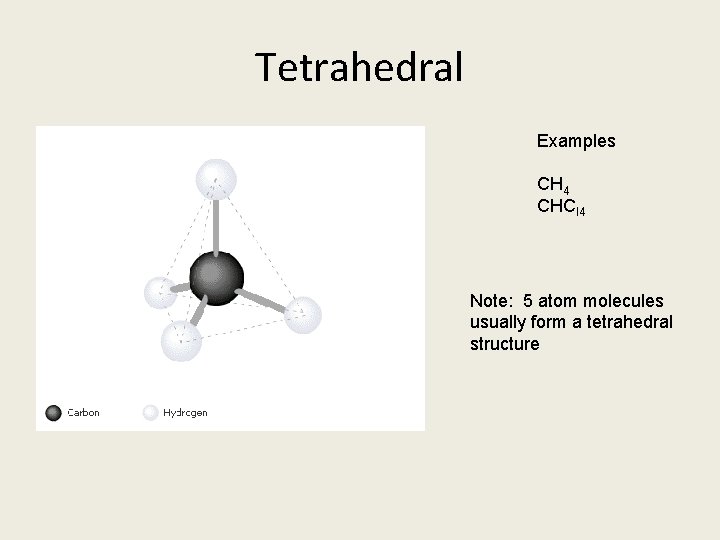
Tetrahedral Examples CH 4 CHCl 4 Note: 5 atom molecules usually form a tetrahedral structure

Trigonal pyramidal Examples Ammonia NH 3

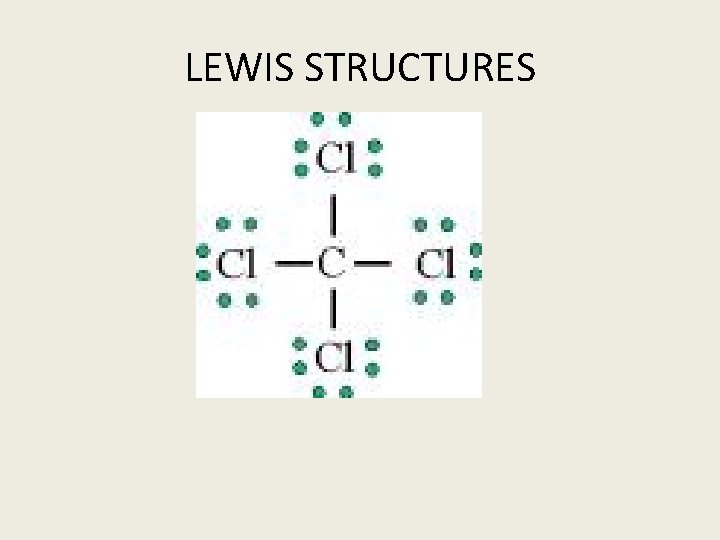
LEWIS STRUCTURES

STEPS IN DRAWING A LEWIS STRUCTURE Determine if the compound is ionic or covalent Covalent 1. Draw the Electron dot (Lewis) structure for each atom in the compound. 1. Determine the shape and arrange atoms accordingly. 1. Place valence electrons around atom and make certain that with the number of V. E. each atom obtains a stable configuration usually 8 with the exception of hydrogen 1. Note only an even number of electrons can exist between two atoms. The maxium number is 6 that is three pairs of electrons.

Ionic compounds 1. Follow the steps as you would for a covalent bond however you will not show sharing but transferring of electrons. 1. Place a bracket around the anion 1. You must indicate charges for both the anion and cation

Unit 6 Chemical Reactions

What should you know? 1. You should be able to identify the reactants and products of the following chemical reactions: a. Synthesis b. Decomposition c. Single replacement d. Double replacement e. Neutralization f. Combustion g. Fermentation h. Addition i. Substitution j. Esterification k. Polymerization Organic Reactions
![2 Chemical reactions are either exothermic or endothermic table I 3 Chemical reactions must 2. Chemical reactions are either exothermic or endothermic [table I] 3. Chemical reactions must](https://slidetodoc.com/presentation_image_h/9d9a098f326f24858e645ae92d8fd24b/image-67.jpg)
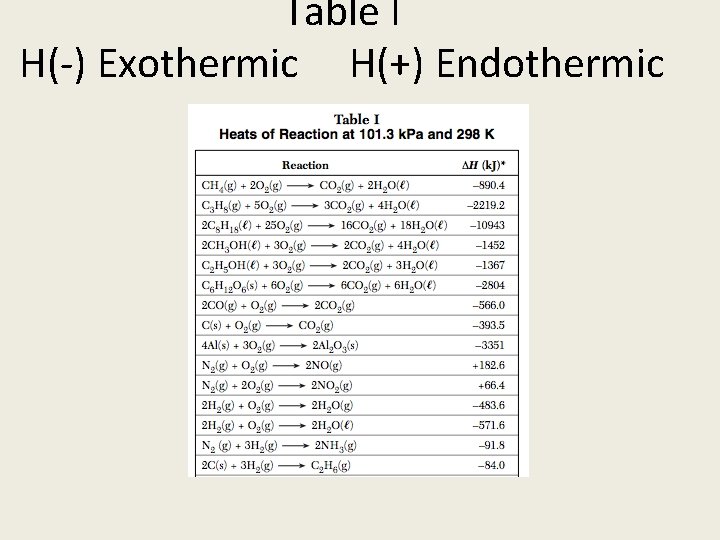
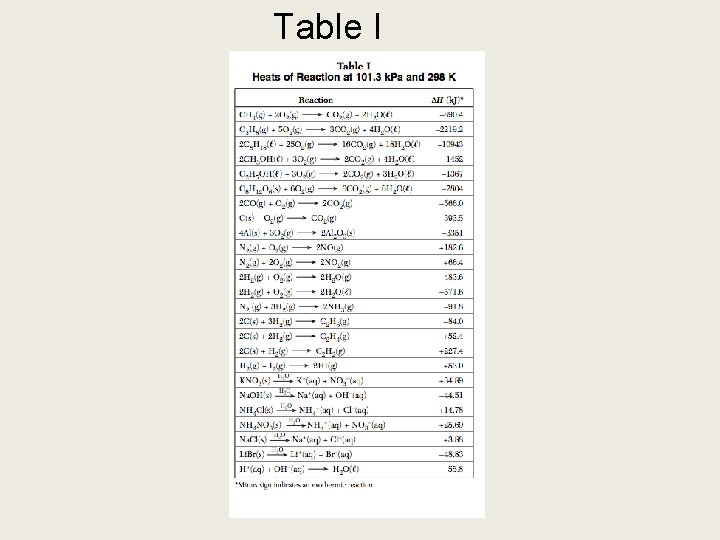
2. Chemical reactions are either exothermic or endothermic [table I] 3. Chemical reactions must be balanced: conservation of mass and charge

Chemical Reaction Sheet

Table I H(-) Exothermic H(+) Endothermic

Unit 7 Solutions

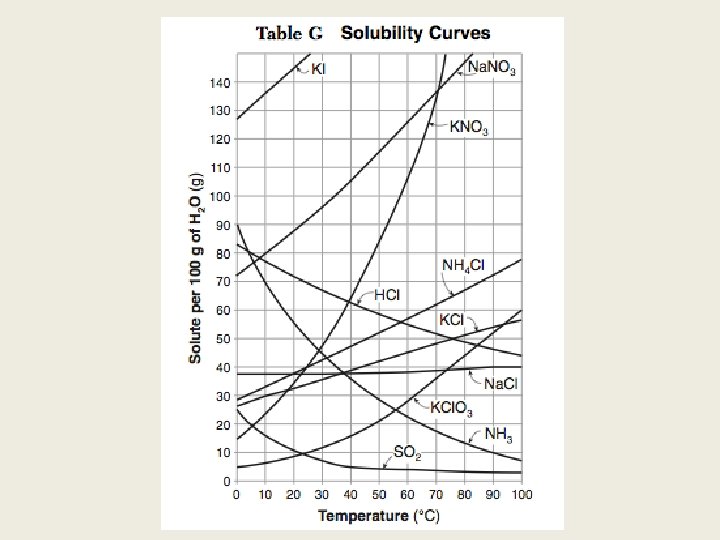
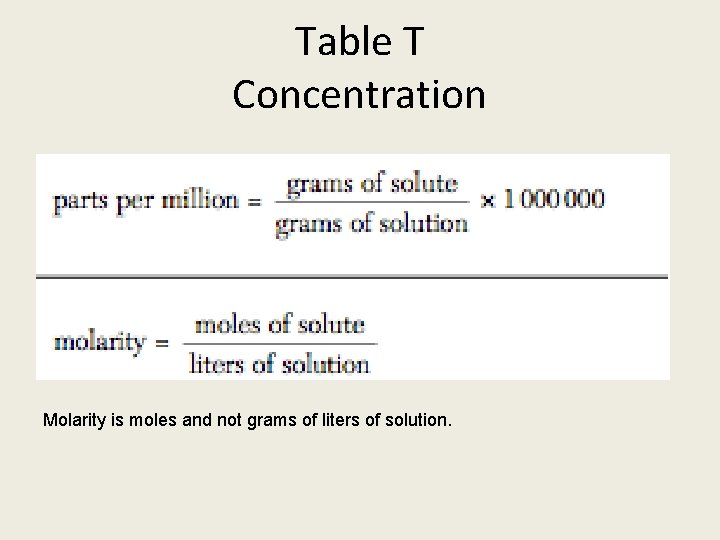
What should you know? 1. Use table F to determine solubility 1. Use table G to determine type of solution 1. Use table T for concentration formulas: molarity and parts per million



Table T Concentration Molarity is moles and not grams of liters of solution.

Unit 8 Acids and Bases

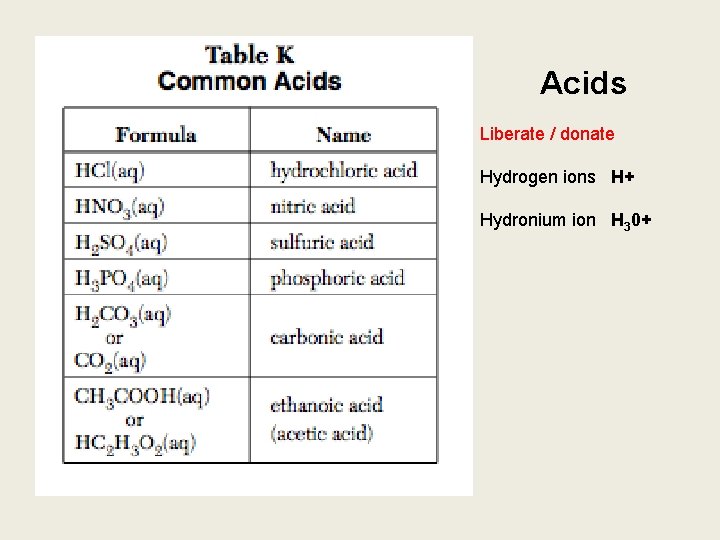
What should I know? 1. Should be able to use table K to identify acids 1. Should be able to use table L to identify bases 1. Should be able to use table M to identify the ranges of the p. H indicators

Acids Liberate / donate Hydrogen ions H+ Hydronium ion H 30+

Bases Arrhenius Definition Liberate OH- in solution Alternative definition Hydrogen acceptor

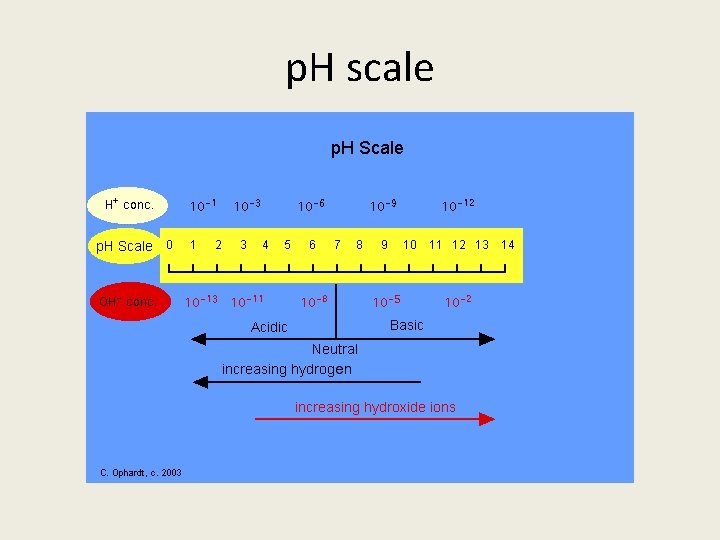
p. H scale


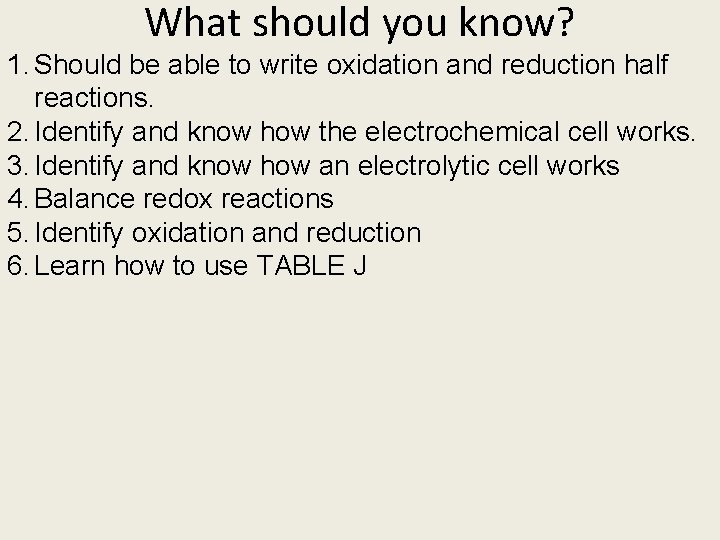
Unit 9 Electrochemistry

What should you know? 1. Should be able to write oxidation and reduction half reactions. 2. Identify and know how the electrochemical cell works. 3. Identify and know how an electrolytic cell works 4. Balance redox reactions 5. Identify oxidation and reduction 6. Learn how to use TABLE J

How does an electrochemical cell work? 2 e. Zn 2 ee. Zn+2 2 e- 2 e. Cu 2 e- Cu+2

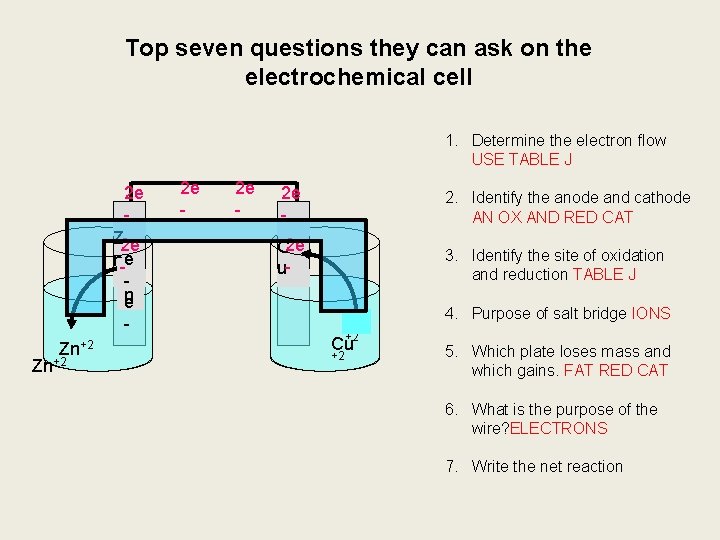
Top seven questions they can ask on the electrochemical cell 1. Determine the electron flow USE TABLE J 2 e Z 2 e n-e -Z n e Zn+2 2 e - 2. Identify the anode and cathode AN OX AND RED CAT C 2 e u- 3. Identify the site of oxidation and reduction TABLE J Cu +2 4. Purpose of salt bridge IONS 5. Which plate loses mass and which gains. FAT RED CAT 6. What is the purpose of the wire? ELECTRONS 7. Write the net reaction

IDENTIFYING OXIDATION AND REDUCTION Lost or gained e. A. H+ + e- --> H B. Cl 2 + 2 e- --> 2 Cl. C. Fe+2 + 2 e- --> Fe D. Cl+5 + 6 e- --> Cl. E. S-2 --> S+4 + 6 e- Gained Lost Oxidized or reduced reduced oxidized

UNIT 11 KINETICS AND EQUILIBRIUM

WHAT SHOULD YOU KNOW? 1. What factors affect the rate of chemical reaction 2. Use the collision theory to explain how the factors affect the rate of a chemical reaction 3. Interpret potential energy diagrams 4. Be able to use table I 5. Explain Le Chatilier’s Principle

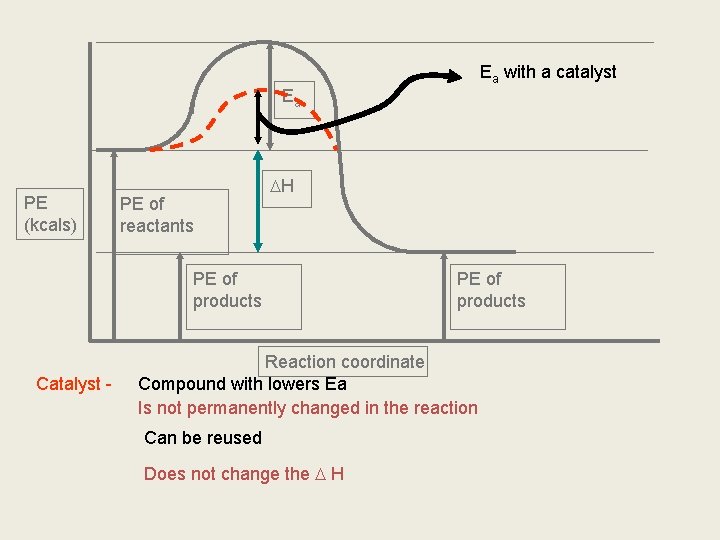
PE of activated complex Ea PE (kcals) H PE of reactants Activated complex PE of products Reaction coordinate Occurs when reactants collide in the proper orientation Bonds are in the process of breaking and forming Very unstable, only lasts a moment Quickly moves to products

Ea with a catalyst Ea PE (kcals) PE of reactants H PE of products Catalyst - PE of products Reaction coordinate Compound with lowers Ea Is not permanently changed in the reaction Can be reused Does not change the H

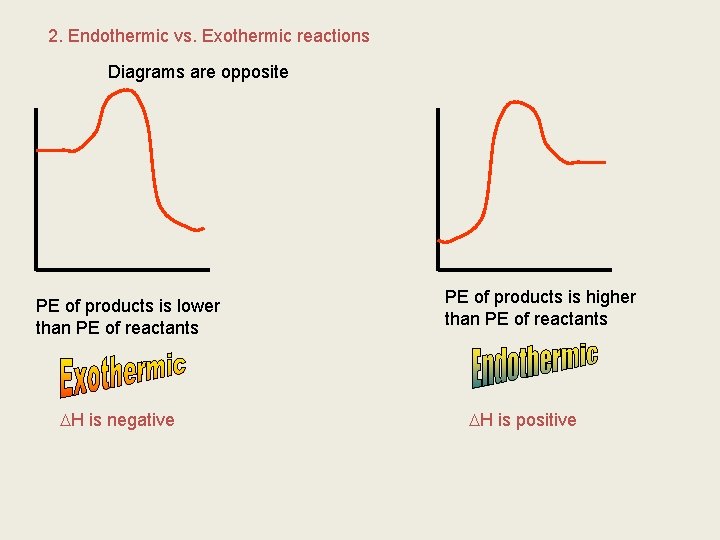
2. Endothermic vs. Exothermic reactions Diagrams are opposite PE of products is lower than PE of reactants H is negative PE of products is higher than PE of reactants H is positive

Table I

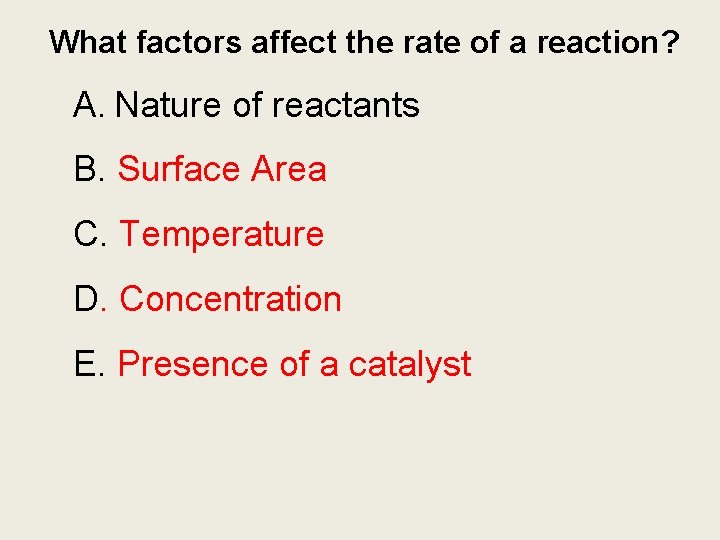
What factors affect the rate of a reaction? A. Nature of reactants B. Surface Area C. Temperature D. Concentration E. Presence of a catalyst

Collision Theory A. Chemists use the collision theory to explain how surface area, temperature, concentration, and presence catalyst.

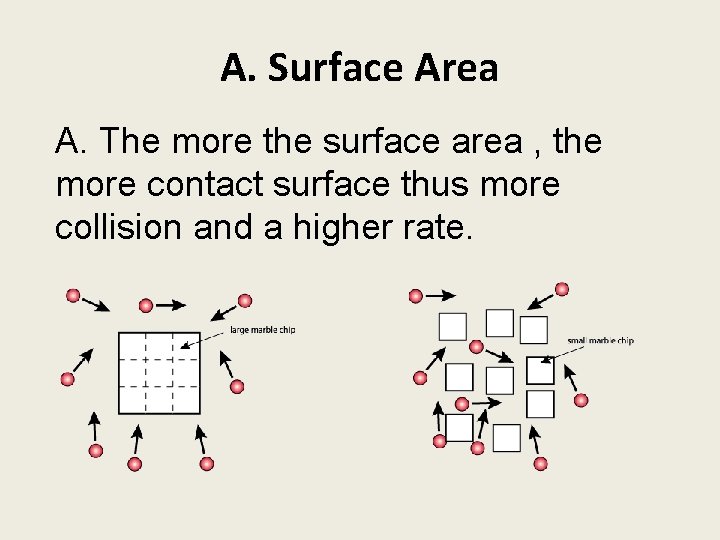
A. Surface Area A. The more the surface area , the more contact surface thus more collision and a higher rate.

B. Temperature A. The higher the temperature (kinetic energy) the higher number of collision which results in a higher rate.

C. Concentration A. Higher the concentration the higher the number of collisions thus the faster the rate

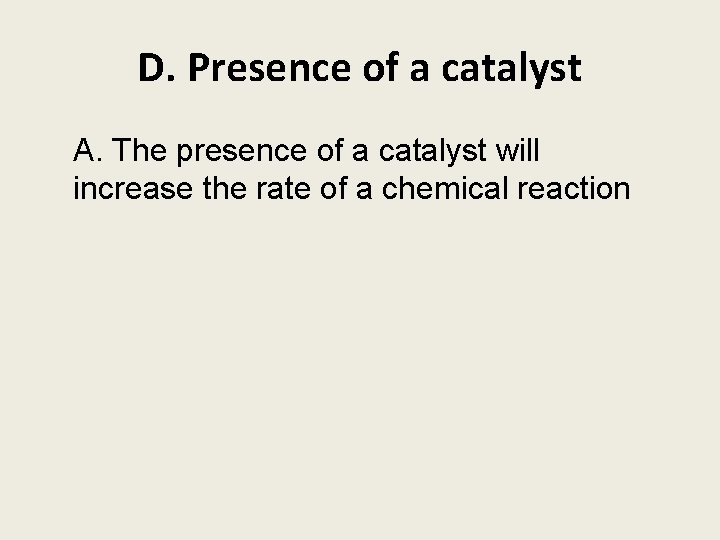
D. Presence of a catalyst A. The presence of a catalyst will increase the rate of a chemical reaction

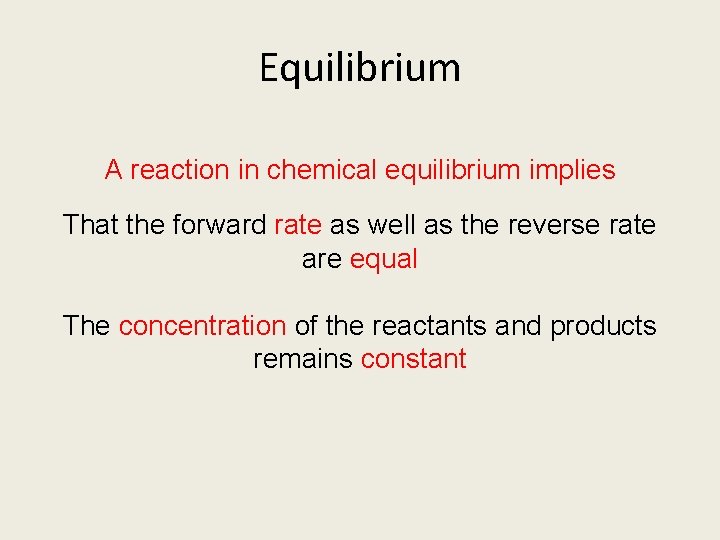
Equilibrium A reaction in chemical equilibrium implies That the forward rate as well as the reverse rate are equal The concentration of the reactants and products remains constant

Le Chatelier’s Principle When a system at equilibrium subjected to stress it will move in the direction of counteract (relieve) the stress.

Factors of stress on equilibrium • Temperature • Concentration • Pressure

Unit 11 Organic Chemistry

What should I know? 1. Learn how to identify organic compounds 2. Learn how to name organic compounds with the use of Tables P, Q, and R 3. Learn how to identify isomers 4. Learn how to identify organic reactions

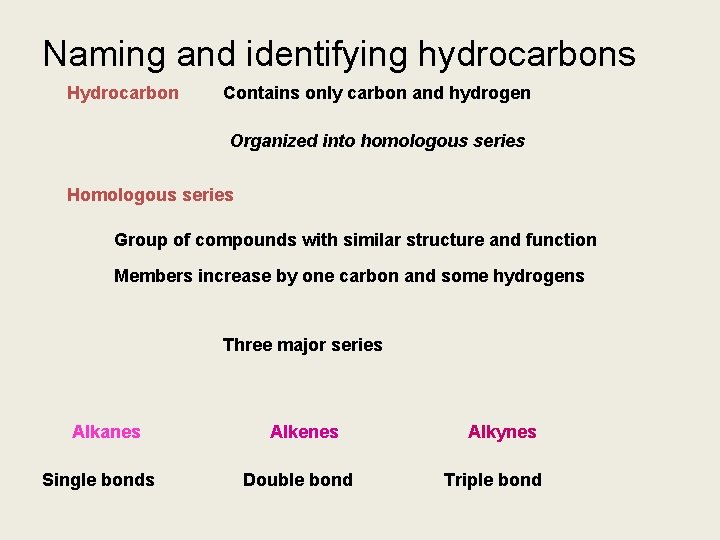
Naming and identifying hydrocarbons Hydrocarbon Contains only carbon and hydrogen Organized into homologous series Homologous series Group of compounds with similar structure and function Members increase by one carbon and some hydrogens Three major series Alkanes Single bonds Alkenes Double bond Alkynes Triple bond



Functional Groups **Atoms or Groups of atoms which will replace Hydrogen in a Hydrocarbon chain. ***TABLE R***

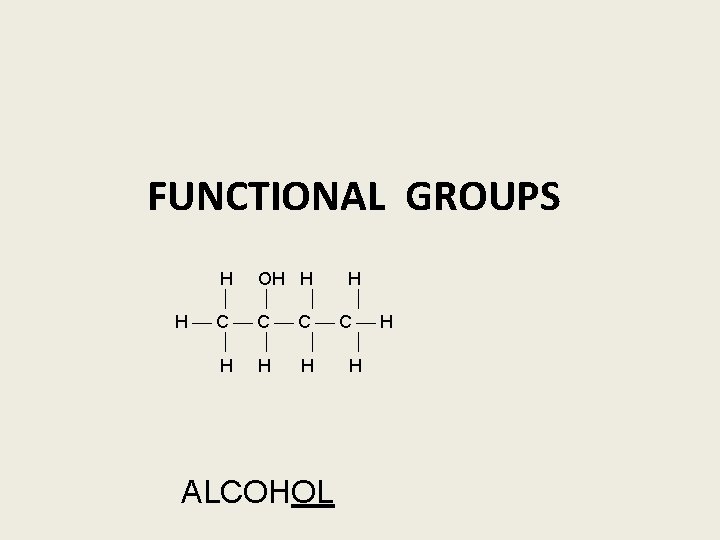
FUNCTIONAL GROUPS H H │ │ H ─ C ─ OH │ │ H H R – OH = ALCOHOL

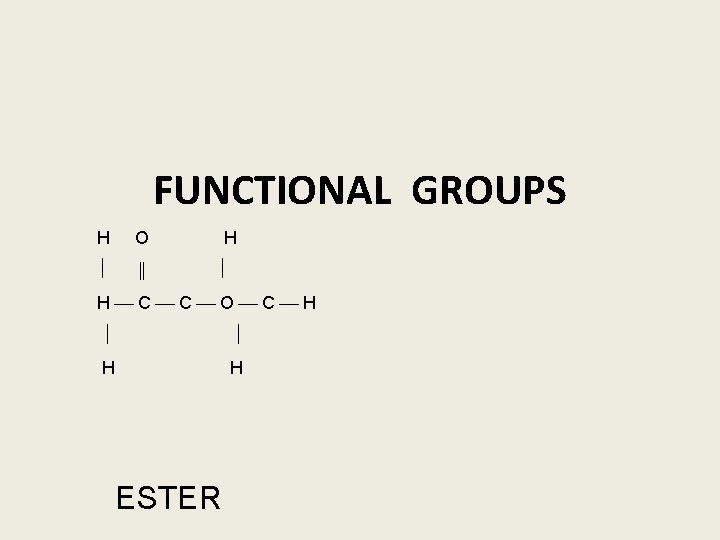
FUNCTIONAL GROUPS H O ‖ H H C C O C H H H ESTER

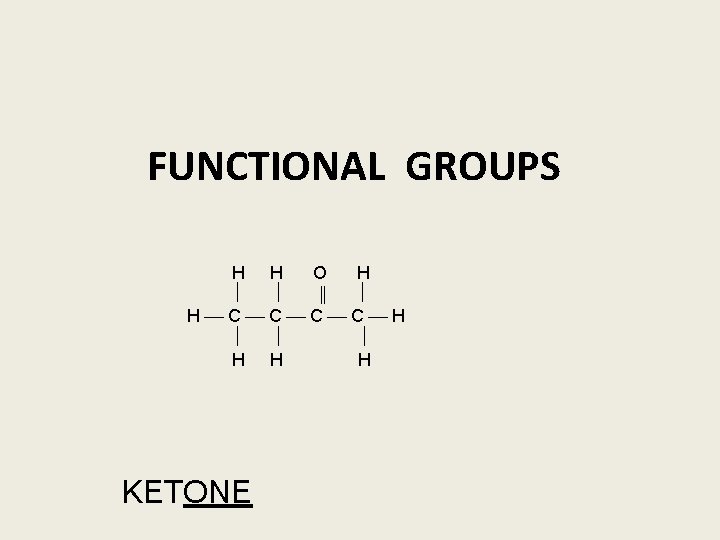
FUNCTIONAL GROUPS H H O H ‖ H C C H H H H KETONE

FUNCTIONAL GROUPS H OH H H H C C H H H ALCOHOL

Organic Reactions

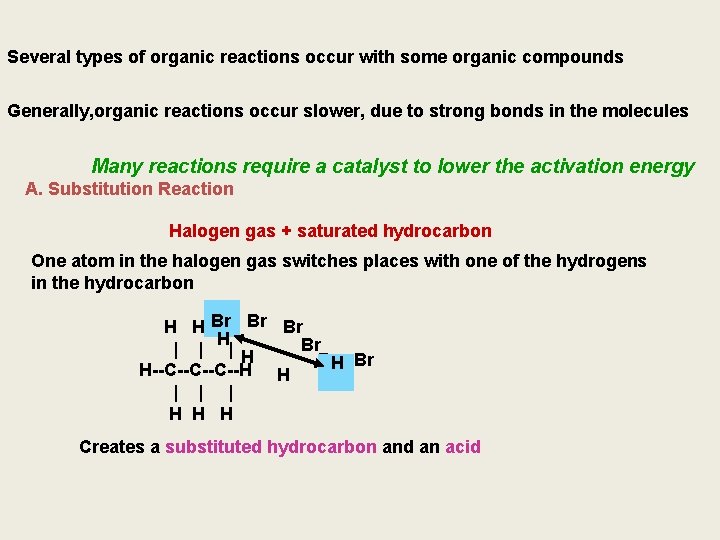
Several types of organic reactions occur with some organic compounds Generally, organic reactions occur slower, due to strong bonds in the molecules Many reactions require a catalyst to lower the activation energy A. Substitution Reaction Halogen gas + saturated hydrocarbon One atom in the halogen gas switches places with one of the hydrogens in the hydrocarbon H H Br Br H Br | | |H Br. H- Br H--C--C--C--H H | | | H H H Creates a substituted hydrocarbon and an acid

B. Addition Reaction Halogen + Unsaturated hydrocarbon Because the double and triple bond is so exposed, it will be the first bond broken when a halogen is added H H H | | / H--C--C==C | open H H H | H--C--C C--H open | H When the double bond breaks, we have two open bonding sites H F F | | F F F--F H--C--C--H | | H H Both of the halogen atoms are added We only have one product!

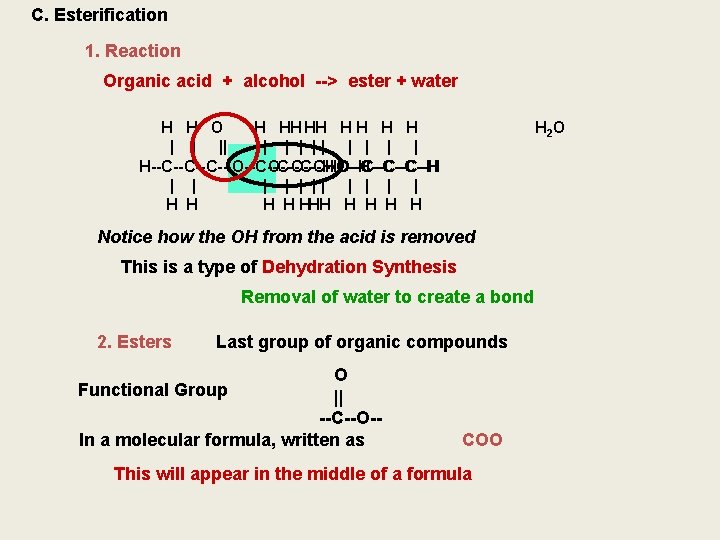
C. Esterification 1. Reaction Organic acid + alcohol --> ester + water H H O H HH HH H H | | || | | | | H--C--C--C--OH O--C--C--C--H HO--C--C--C--H | | | || | | H H HHH H H 2 O Notice how the OH from the acid is removed This is a type of Dehydration Synthesis Removal of water to create a bond 2. Esters Last group of organic compounds O Functional Group || --C--O-In a molecular formula, written as COO This will appear in the middle of a formula

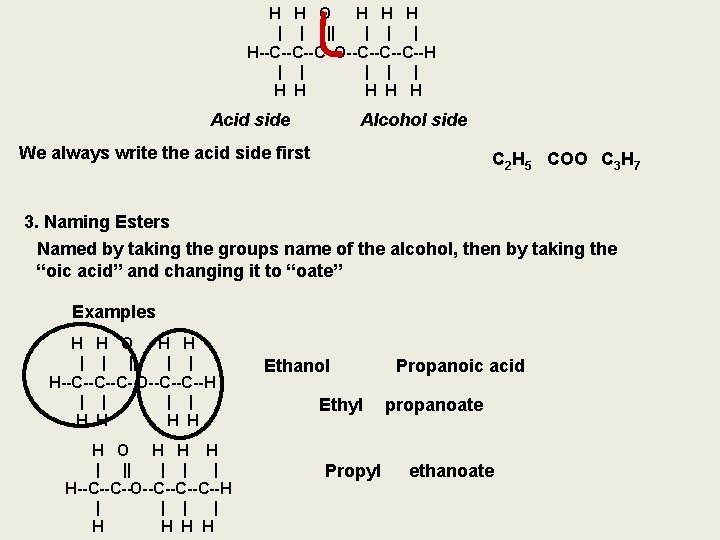
H H O H H H | | | | H--C--C--C--O--C--C--C--H | | | H H H Acid side Alcohol side We always write the acid side first C 2 H 5 COO C 3 H 7 3. Naming Esters Named by taking the groups name of the alcohol, then by taking the “oic acid” and changing it to “oate” Examples H H O H H | | | H--C--C--C--O--C--C--H | | H H H O H H H | || | H--C--C--O--C--C--C--H | | H H Ethanol Ethyl Propanoic acid propanoate ethanoate

D. Fermentation Reaction Very complex reaction Some living organisms break down sugar to get energy. Some of these organisms cause sugar to form ethanol C 6 H 12 O 6 Sugar zymase 2 C 2 H 5 OH + 2 CO 2 Ethanol + carbon dioxide Requires an enzyme Xymase

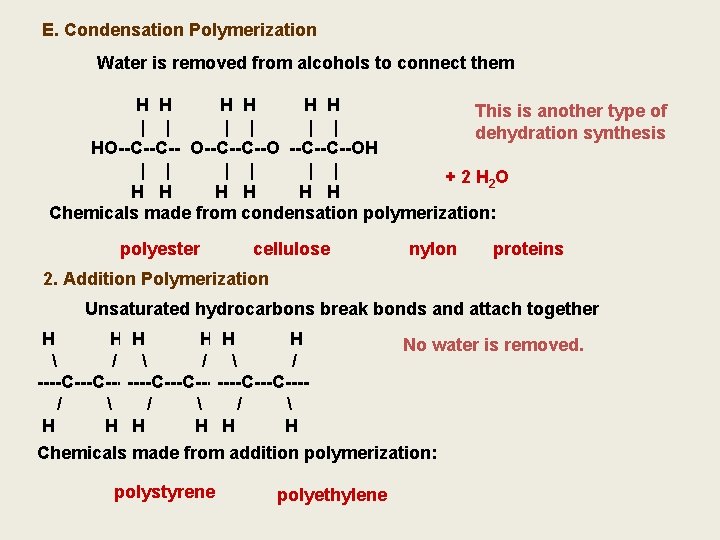
E. Condensation Polymerization Water is removed from alcohols to connect them H HH H H H This is another type of | || | | | | dehydration synthesis OH H HO--C--C--OH HO--C--C--O --C--C--OH | || | | | + 2 H 2 O H HH H H H H Chemicals made from condensation polymerization: polyester cellulose nylon proteins 2. Addition Polymerization Unsaturated hydrocarbons break bonds and attach together HH HHH HH \ / / \ / // / / / ----C---C-------C---C---C==C / // \/ // \ / / H HH HH HH No water is removed. Chemicals made from addition polymerization: polystyrene polyethylene

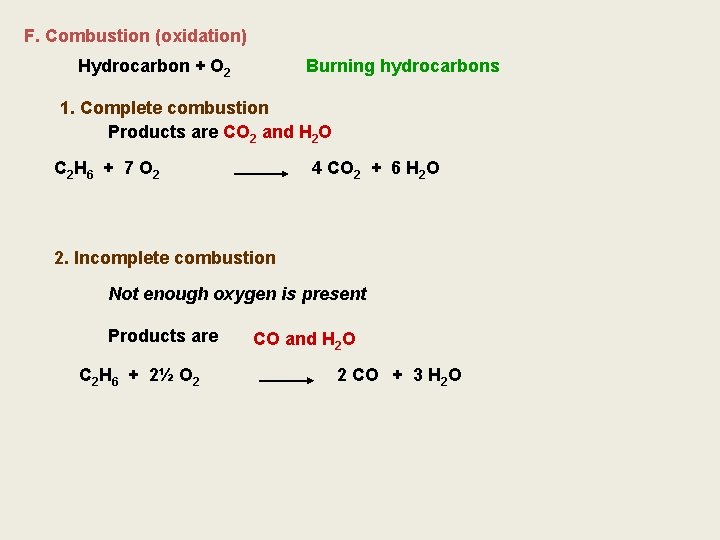
F. Combustion (oxidation) Hydrocarbon + O 2 Burning hydrocarbons 1. Complete combustion Products are CO 2 and H 2 O C 2 H 6 + 7 O 2 4 CO 2 + 6 H 2 O 2. Incomplete combustion Not enough oxygen is present Products are C 2 H 6 + 2½ O 2 CO and H 2 O 2 CO + 3 H 2 O

UNIT 12 NUCLEAR CHEMISTRY

WHAT SHOULD I KNOW? 1. Should be able to describe the different forms of nuclear decay: alpha, beta, and gamma 2. Should be able to balance nuclear reactions 3. Should be able to determine the half life of an atom 4. Should be able to use table N and O

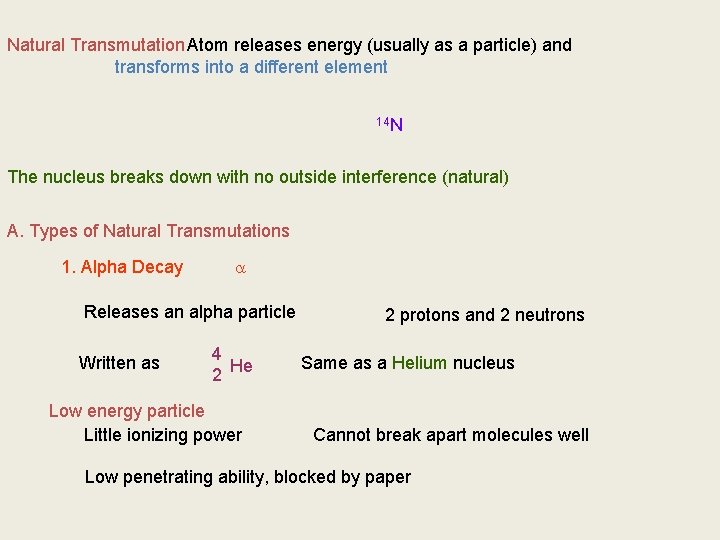
Natural Transmutation Atom releases energy (usually as a particle) and transforms into a different element 14 14 N C The nucleus breaks down with no outside interference (natural) A. Types of Natural Transmutations 1. Alpha Decay Releases an alpha particle Written as 4 He 2 Low energy particle Little ionizing power 2 protons and 2 neutrons Same as a Helium nucleus Cannot break apart molecules well Low penetrating ability, blocked by paper

2. Beta particle b - Releases a beta particle and energy from the nucleus - Actually it is an electron 0 -1 e No noticeable mass Opposite charge of a proton - a neutron breaks down into an electron and a proton - the electron is then released from the nucleus Moves faster than alpha, almost to the speed of light More damaging, - Higher penetrating abillity and ionizing ability blocked by thin sheets of lead 3. (gamma decay) No particle, just a release of energy Energy is similar to high energy x-rays Very high ionizing ability and penetrating ability Can penetrate several cm of lead, blocked by thick lead

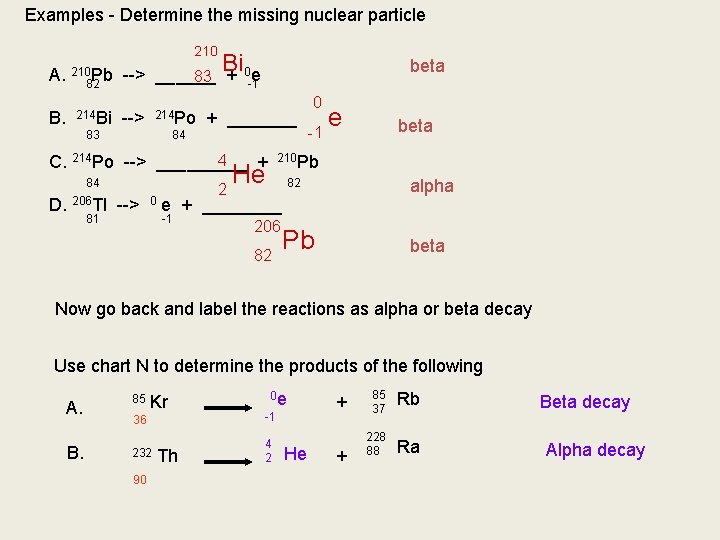
Chart H - Lists types of decays for many nulcei B. Writing Nuclear Reactions We can determine what particles are present by what is left after the decay 222 86 226 88 Ra --> Since we lost 4 nuclear particles Since we lost 2 protons 4 2 Rn 86 He + _____ We have 222 left We have 86 left To determine the identity, look at the atomic number and match it to the periodic table

Examples - Determine the missing nuclear particle 210 Bi beta A. 21082 Pb --> ______ 83 + 0 -1 e B. 214 Bi 83 214 Po --> 84 + _______ 4 C. 214 Po --> _____ + 84 D. 206 Tl --> 0 81 2 0 -1 e beta 210 Pb He alpha 82 e + ____ -1 206 82 Pb beta Now go back and label the reactions as alpha or beta decay Use chart N to determine the products of the following A. B. 85 Kr 0 e 36 -1 232 4 2 90 Th He + + 85 37 Rb 228 88 Ra Beta decay Alpha decay

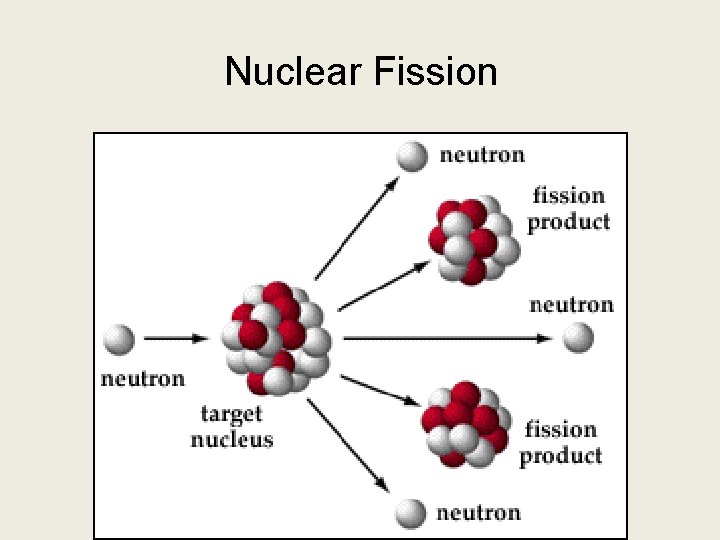
Nuclear Fission




 Subatomic particles can usually pass undeflected
Subatomic particles can usually pass undeflected Understanding jim crow (setting the setting)
Understanding jim crow (setting the setting) Physical setting of to kill a mockingbird
Physical setting of to kill a mockingbird Milli micro nano
Milli micro nano Secondary quantities
Secondary quantities Mega prefix
Mega prefix Kilo mega giga tera
Kilo mega giga tera Engeneering notation
Engeneering notation Pt. cakrawala mega indah
Pt. cakrawala mega indah Kilo mega giga tera peta
Kilo mega giga tera peta Mitä pilvipalvelut ovat
Mitä pilvipalvelut ovat Mega.ruz
Mega.ruz Km to m
Km to m Megadyne mega soft
Megadyne mega soft Mega personell
Mega personell Milli deci centi
Milli deci centi Holoprosencéphalie échographie
Holoprosencéphalie échographie Mega giga tera peta
Mega giga tera peta 7/8 as a decimal
7/8 as a decimal Engineering notation
Engineering notation Mega giga tera peta
Mega giga tera peta Zadnji mega žur
Zadnji mega žur Mega giga tera
Mega giga tera Milli centi deci chart
Milli centi deci chart Mega giga tera peta
Mega giga tera peta Taylor overby mega
Taylor overby mega Biodiversity introduction
Biodiversity introduction Kilo mega giga tera
Kilo mega giga tera Pico number
Pico number Arduino nano wikipedia
Arduino nano wikipedia Kilo mega giga tera
Kilo mega giga tera Teodora marinkovic
Teodora marinkovic Mikro makro ja megamaailm
Mikro makro ja megamaailm Megacity definition geography
Megacity definition geography Mega molecular evolutionary genetics analysis
Mega molecular evolutionary genetics analysis Kilo hekto deci centi milli
Kilo hekto deci centi milli Juan pablo laguna la mega
Juan pablo laguna la mega Mega man robot master contest
Mega man robot master contest Méga puissance
Méga puissance Alimentazione arduino mega
Alimentazione arduino mega Niveis.virtua
Niveis.virtua Mega image botosani
Mega image botosani Medpol mega
Medpol mega Ottawit mega stores inc
Ottawit mega stores inc Mega stopper
Mega stopper Mega image romania
Mega image romania Mega goal 4
Mega goal 4 Valentina 94 mega
Valentina 94 mega Mega tree controller
Mega tree controller Mega kilo deci centi milli micro nano
Mega kilo deci centi milli micro nano Deci centi mili micro nano pico
Deci centi mili micro nano pico Leemos sobre el perú un país festivo y mega diverso
Leemos sobre el perú un país festivo y mega diverso 666420
666420 See think wonder
See think wonder Mega ease
Mega ease At mega 328
At mega 328 Notacion cientifica kilo mega giga tera
Notacion cientifica kilo mega giga tera Arduino interrupt timer esempio
Arduino interrupt timer esempio Cpu mega
Cpu mega Mega reto
Mega reto Mega 128
Mega 128 Mega equals
Mega equals Kuvvet makinaları
Kuvvet makinaları Sifat mega
Sifat mega Arduino mega pin diagram
Arduino mega pin diagram Mega goal 4 expansion
Mega goal 4 expansion Mega phylogenetics
Mega phylogenetics People laughing
People laughing Mega juli
Mega juli Veliciny a ich jednotky
Veliciny a ich jednotky Mega vesica
Mega vesica Mega kowalczyk biomasa
Mega kowalczyk biomasa Mega projects in kerala
Mega projects in kerala Mision imposible 6 mega
Mision imposible 6 mega Mega tracking
Mega tracking Sarah snyder mega
Sarah snyder mega Mega open source
Mega open source Mega actor
Mega actor Xxapple mega
Xxapple mega Kayla mari mega
Kayla mari mega Mega tourism
Mega tourism Personalsin xxx
Personalsin xxx Mega crisis definition
Mega crisis definition Mega t 15 deluxe price
Mega t 15 deluxe price Long lot survey system ap human geography
Long lot survey system ap human geography Accannarsi
Accannarsi Chapter review motion part a vocabulary review answer key
Chapter review motion part a vocabulary review answer key Writ of certiorari ap gov example
Writ of certiorari ap gov example Nader amin-salehi
Nader amin-salehi Narrative review vs systematic review
Narrative review vs systematic review Narrative review vs systematic review
Narrative review vs systematic review Glencoe physical science chapter 2 review answers
Glencoe physical science chapter 2 review answers Physical science eoc review
Physical science eoc review Physical science eoc review
Physical science eoc review Chapter 4 work and energy section 1 work and machines
Chapter 4 work and energy section 1 work and machines Physical science chapter 14 review
Physical science chapter 14 review Chapter 15 review physical science
Chapter 15 review physical science Chapter 4 review physical science
Chapter 4 review physical science Chapter 14 review physical science
Chapter 14 review physical science Physical science final exam study guide
Physical science final exam study guide Physical science chapter 5 review
Physical science chapter 5 review Physical science chapter 6 review answers
Physical science chapter 6 review answers Physical review a
Physical review a Michael s fuhrer
Michael s fuhrer Chapter 16 review physical science
Chapter 16 review physical science Chemistry semester 2 review unit 12 thermochemistry
Chemistry semester 2 review unit 12 thermochemistry Chemistry chapter 9 stoichiometry
Chemistry chapter 9 stoichiometry Trinitrogen monosulfide formula
Trinitrogen monosulfide formula Chapter 14 review acids and bases section 1
Chapter 14 review acids and bases section 1 Modern chemistry chapter 13 review answers
Modern chemistry chapter 13 review answers Modern chemistry chapter 12 review answers
Modern chemistry chapter 12 review answers Grade 9 chemistry review
Grade 9 chemistry review Geometry sol review packet
Geometry sol review packet Chemistry unit review answer key
Chemistry unit review answer key Chemistry sol review
Chemistry sol review Chapter 8 review chemical equations and reactions
Chapter 8 review chemical equations and reactions Chapter 7 organic chemistry
Chapter 7 organic chemistry Chapter 22 review organic chemistry section 1 answers
Chapter 22 review organic chemistry section 1 answers Ap chemistry equilibrium review
Ap chemistry equilibrium review Ap chemistry big idea 2 review answers
Ap chemistry big idea 2 review answers Chemistry sol review
Chemistry sol review Chemistry sol review
Chemistry sol review Chemistry sol review
Chemistry sol review Chemistry midterm review
Chemistry midterm review Chemistry math review
Chemistry math review Chemical bonding regents questions
Chemical bonding regents questions Ap chemistry midterm exam
Ap chemistry midterm exam Annual review of analytical chemistry
Annual review of analytical chemistry Chemistry semester 1 exam review answers
Chemistry semester 1 exam review answers Modern chemistry chapter 15
Modern chemistry chapter 15