Meeting with the South Korean Pharmaceutical Industry delegation


![Imports and exports of pharmaceuticals [in 2007] S. African pharmaceutical market: US$ 2. 6 Imports and exports of pharmaceuticals [in 2007] S. African pharmaceutical market: US$ 2. 6](https://slidetodoc.com/presentation_image/ef4a9c502342db25c704fe847650aebd/image-4.jpg)














- Slides: 26

Meeting with the South Korean Pharmaceutical Industry delegation to South Africa 23 February 2009 The pharmaceutical sector in the South African economy ● Essential facts & figures ● Economic incentives for investors

An outline of the presentation 1. Essential facts & figures concerning the pharmaceutical sector in the S. African economy. (a) Trade balance, imports and exports, trade dynamics. (b) Structure of the industry, key players. (c) Pharmaceutical industry among the “priority sectors” in Government’s industrial policy. 2. Legislative and regulatory environment. (a) South African patent law. (b) Registration of medicines and manufacturers. (c) Price control of medicines. 3. Economic incentives. (a) Tax incentives for investors. (b) Incentives for small & medium investment projects (EIP-MIP). (c) Research & development (R&D) incentives.

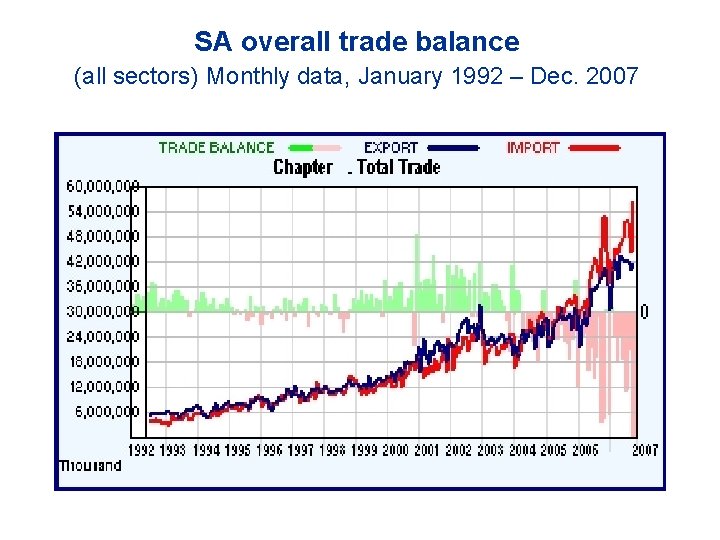
SA overall trade balance (all sectors) Monthly data, January 1992 – Dec. 2007
![Imports and exports of pharmaceuticals in 2007 S African pharmaceutical market US 2 6 Imports and exports of pharmaceuticals [in 2007] S. African pharmaceutical market: US$ 2. 6](https://slidetodoc.com/presentation_image/ef4a9c502342db25c704fe847650aebd/image-4.jpg)
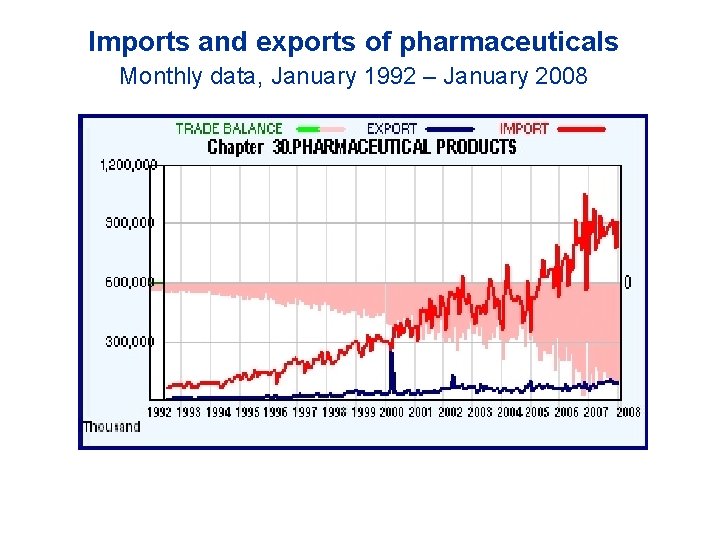
Imports and exports of pharmaceuticals [in 2007] S. African pharmaceutical market: US$ 2. 6 billion (= 18 billion Rands) at Ex Factory price level. ◇ Local production: US$ 660 million ◆ Imports: US$ 1. 97 billion (including active pharmaceutical ingredients, intermediates, other materials). - Imports under Customs Tariff Chapter 30: 10. 45 billion Rands, exports 1 billion Rands (NB: This excludes active pharmaceutical ingredients which are almost 100% imported). ◆ Overall, trade deficit in the healthcare goods sector (pharmaceuticals, medical devices and medical diagnostics) was over 22 billion Rands in 2007.

Pharmaceutical markets: South Africa vs. other sub-Saharan African countries (World Bank report)

Imports and exports of pharmaceuticals Monthly data, January 1992 – January 2008

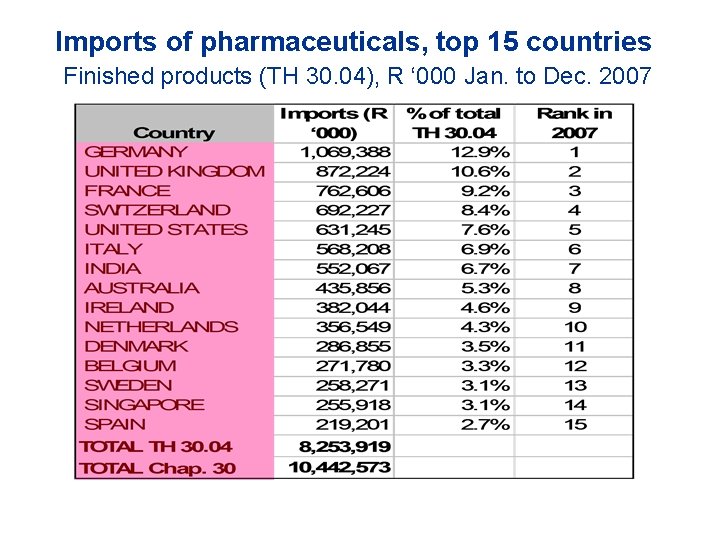
Imports of pharmaceuticals, top 15 countries Finished products (TH 30. 04), R ‘ 000 Jan. to Dec. 2007

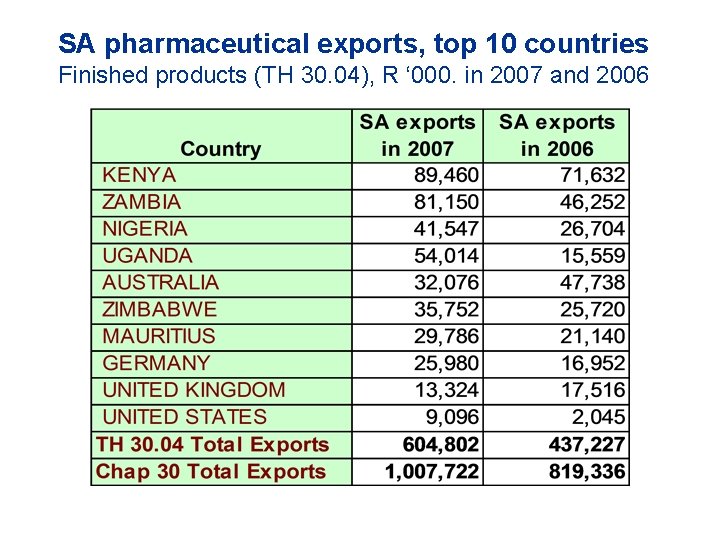
SA pharmaceutical exports, top 10 countries Finished products (TH 30. 04), R ‘ 000. in 2007 and 2006

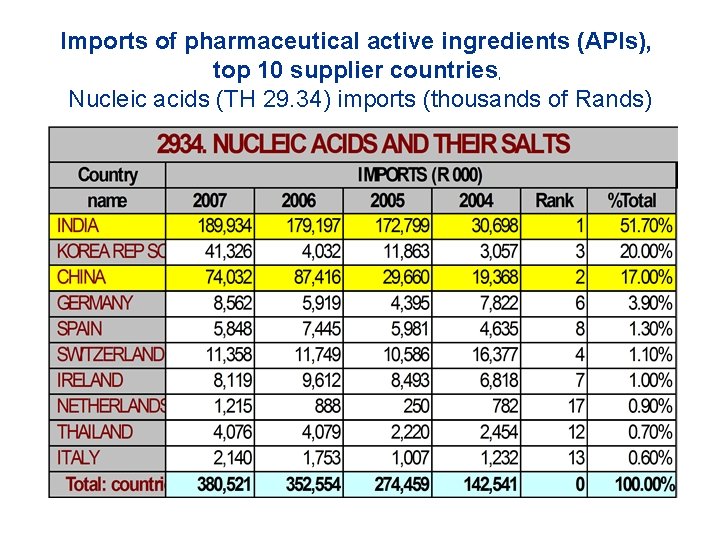
Imports of pharmaceutical active ingredients (APIs), top 10 supplier countries, Nucleic acids (TH 29. 34) imports (thousands of Rands)

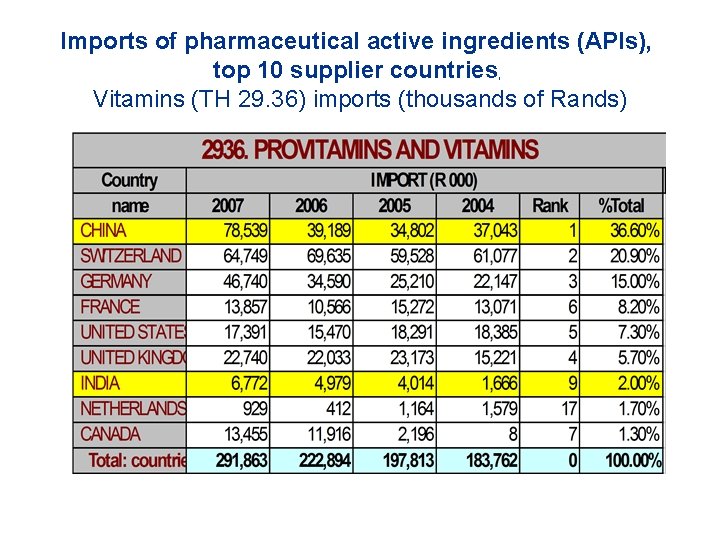
Imports of pharmaceutical active ingredients (APIs), top 10 supplier countries, Vitamins (TH 29. 36) imports (thousands of Rands)

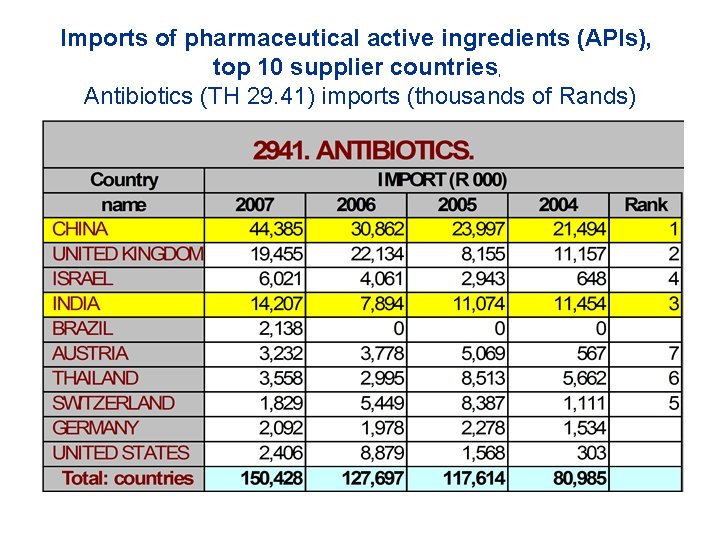
Imports of pharmaceutical active ingredients (APIs), top 10 supplier countries, Antibiotics (TH 29. 41) imports (thousands of Rands)

Government’s programme of economic support for the pharmaceutical industry. ► DTI’s National Industrial Policy Framework (NIPF) and Industrial Policy Action Plan (IPAP). The pharmaceutical industry is among the “priority sectors” targeted for economic intervention. ► Cabinet of Ministers endorsed NIPF and IPAP in 2007 ► Key action plan: - Designation of production of anti-retroviral medicines (ARVs) - as a strategic industry, Expanding local capabilities to increase domestic production of ARVs and other essential medicines, Feasibility studies: Manufacture of active pharmaceutical ingredients (APIs): for ARVs, anti-TB, biologicals and other.

The South African healthcare and pharmaceutical sectors South African national healthcare is divided into 2 sectors: Public sector (responsible for 40 million people, with no medical insurance) Private sector (caters for 7 million people, of whom 3 million are Black and 3 million White, most of them covered by private medical insurance) Vast disparities between the private and public sectors, in terms of funding, quality of facilities and services.

The South African healthcare and pharmaceutical sectors (2) South African total healthcare facilities: 852 hospitals and clinics with 137, 000 hospital beds, 30, 750 physicians, 193, 100 nurses and 10, 820 pharmacists (2006 data). Three hospital groups in the private sector: Afrox, Netcare and Medi-Clinic; jointly 22, 000 hospital beds. National spending on healthcare: 8. 2% to 8. 5% of the GDP (total US$ 15. 9 billion, i. e. average US$ 331 per capita, in 2005). Annual spending R 695 per capita in public sector, vis -à-vis R 5, 714 in the private sector (2001 data).

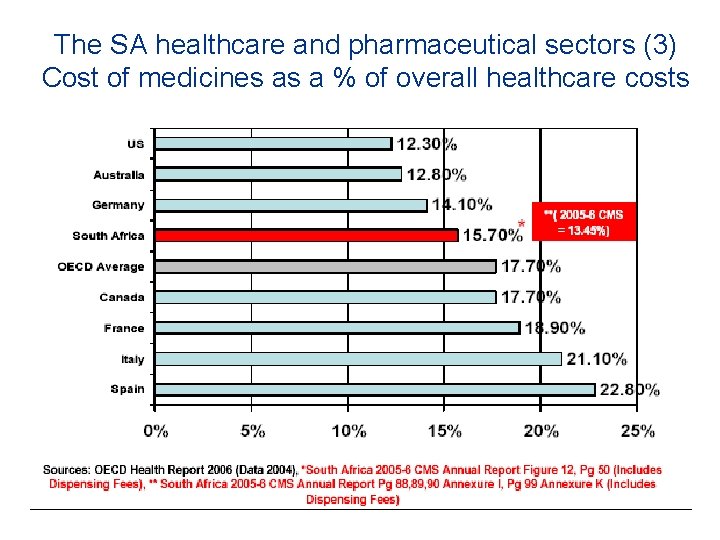
The SA healthcare and pharmaceutical sectors (3) Cost of medicines as a % of overall healthcare costs

The South African pharmaceutical sector The South African pharmaceutical market level was US$ 2. 6 billion (18 billion Rands) in 2007 (0. 3% of the global market, at Ex Factory prices). The SA pharmaceutical market is divided into: ◇ Private (value R 14. 2 bn and 257 million units (mostly branded and patented medicines) ◇ Public (R 3. 7 billion, 158 million units (mostly generics)

The South African pharmaceutical sector (2) Largest companies: • SA-owned: Aspen-Pharmacare and Adcock. Ingram (manufacturers of generic drugs) ⊳ Aspen-Pharmacare R 4 billion sales in 2007 (US$ 600 million) ⊳ Adcock-Ingram R 2. 8 billion sales. • Multi-national corporations: Sanofi- Aventis, Pfizer, Merck (MSD), Glaxo-Smith Kline (GSK), Sandoz, Novartis, Roche, Astra-Zeneca and Johnson & Johnson • Indian companies: Cipla-Medpro and Ranbaxy.

The SA pharmaceutical sector (3) Market share of top 15 companies (private sector)

The legal and regulatory environment in SA 1. IP protection. - The SA Patents’ law is fully TRIPS compliant; protection 20 years from the date of filing, no extension. No examination of patent application. SA “Bolar” (registration may start before patent expiry) 2. Amendment Bill to the National Health Act and the Medicines Control Act (in Parliament). 3. Price control of medicines - Applies only to the private sector, Single Exit Prices (SEP), Annual price increases, International price benchmarking. 4. Registration of medicines.

Economic incentives for investors Tax incentives (tax deductions / tax allowances) ● Strategic Industrial Projects – “SIP” – for large investment projects, was available 2003 to 2008. • 900 million Rands allocated to pharmaceutical projects under SIP (Roche, Aspen and Adcock). ● New tax incentives (available from April 2009) Objectives: To increase the competitiveness of South African manufacturing sectors by promoting / incentivising investment in advanced technologies (new products), equipment and skills. ▪ Greenfield investment projects, ▪ Brownfield / expansion investment projects, and ▪ Substantial upgrade projects (recapitalization / replacement of aged / old technology equipment.

Economic incentives for investors Tax incentives (2) • Types of qualifying projects and limits: Greenfield projects: investment minimum R 200 million in industrial assets (max. R 1. 5 billion). ▪ Brownfield / expansion projects: investment R 30 million or 25% of the value of existing assets. ▪ Substantial upgrade projects (recapitalization / replacement of aged / old technology equipment. R 30 mill or 25% of the value of existing assets. • 2 levels of incentives for: (I) Preferred status projects and (II) Ordinary (qualifying) status projects. ▪

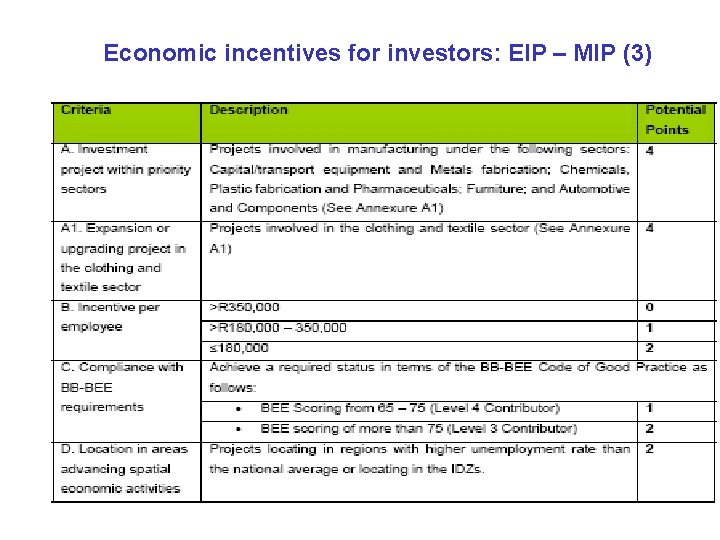
Economic incentives for investors: Enterprise Investment Programme – Manufacturing Investment Programme (EIP – MIP) · Time-lines: EIP – MIP launched in July 2008. It will remain available for the next 6 years, until 2014. · EIP – MIP is an investment incentive to stimulate investment growth, in line with the Government’s National Industrial Policy Framework. · Aim: To enhance the sustainability of manufacturing investment projects by SME’s and to support large-to-medium investment projects in manufacturing that would otherwise not be established without the grant. · Projects above R 5 million qualify for a tax-free investment grant between 30% and 15% of their qualifying investment costs. The grant is calculated on a regressive scale. The grant is maximum 30 million Rands. It is paid over two years.

Economic incentives for investors: EIP-MIP (2) General principles for using the EIP - MIP incentive: · Filling funding gaps where there is no sufficient equity for the capitalization of the project; · Filling funding gaps where cash flows cannot support more third party debt; · Influencing location of the project in favour of South Africa in cases where the investor is considering other countries for the project; · Satisfying the investor’s investment evaluation criteria (such as the Internal Rate of Return - IRR or Net Present Value - NPV). (NB: The applicants must demonstrate how the EIP-MIP grant is necessary for their project to proceed. Projects are expected to explore other sources of funding before seeking the EIP-MIP).

Economic incentives for investors: EIP – MIP (3)

Opportunities for the pharmaceutical industry in South Africa • Growing black middle class and their focus on family healthcare • Increasing level of overall welfare of the SA population, making quality healthcare affordable for larger numbers of people, • Increased access to medical insurance schemes, • Expansion of private and public hospital base, • Rapid urbanization, resulting in a visible move from “traditional African remedies” to Western medicines, ► (unfortunately) the HIV / AIDS and TB epidemics,

THE DEPARTMENT OF TRADE AND INDUSTRY www. thedti. gov. za Contact address: AKudlinski@thedti. gov. za Tel +27 12 3941384; cell +27 83 276 0376
 Korean coat of arms
Korean coat of arms Fraud in pharmaceutical industry
Fraud in pharmaceutical industry Good manufacturing practices in pharmaceutical industry
Good manufacturing practices in pharmaceutical industry Enterprise risk management pharmaceutical industry
Enterprise risk management pharmaceutical industry Fire hazards in pharmaceutical industry
Fire hazards in pharmaceutical industry Rutgers pharmacy fellowship
Rutgers pharmacy fellowship Toc in pharmaceutical industry
Toc in pharmaceutical industry Customer satisfaction in pharmaceutical industry
Customer satisfaction in pharmaceutical industry Pharma due diligence checklist
Pharma due diligence checklist Importance of quality in pharmaceutical industry
Importance of quality in pharmaceutical industry Concurrent validation in pharmaceutical industry
Concurrent validation in pharmaceutical industry What is meeting and types of meeting
What is meeting and types of meeting For todays meeting
For todays meeting Meeting objective
Meeting objective What is meeting and types of meeting
What is meeting and types of meeting Old south vs new south streetcar named desire
Old south vs new south streetcar named desire Sae government industry meeting 2019
Sae government industry meeting 2019 Sae government industry meeting 2019
Sae government industry meeting 2019 Safi south africa
Safi south africa Leadership delegation and empowerment
Leadership delegation and empowerment Supervision guidelines for nursing and midwifery
Supervision guidelines for nursing and midwifery Delegation event model adalah
Delegation event model adalah Delegation of financial power
Delegation of financial power Contoh participative leadership
Contoh participative leadership Microsoft kerberos configuration manager for sql server
Microsoft kerberos configuration manager for sql server Delegation continuum
Delegation continuum Delegation of authority framework
Delegation of authority framework