Meeting with the SA Pharmaceutical Industry Department of
















- Slides: 25

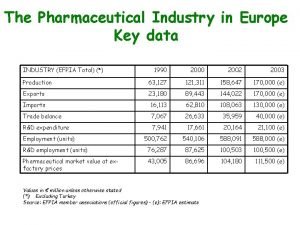
Meeting with the SA Pharmaceutical Industry Department of Health 4 September 2008 DTI’s new economic incentives Part 1: The pharmaceutical sector in the SA economy Essential facts & figures

An outline of the presentation 1. 2. Key facts & figures concerning the pharmaceutical sector in the South African economy. - Trade balance and trade dynamics - South Africa vs. the rest of Africa Pharmaceutical industry among the “priority sectors” in the DTI’s NIPF and IPAP. - Formulation of industrial strategy for the SA pharmaceutical sector. - Phase I: ARV APIs pre-feasibility study - Phase II: other key APIs, advanced formulation, biologicals 3. New economic incentives

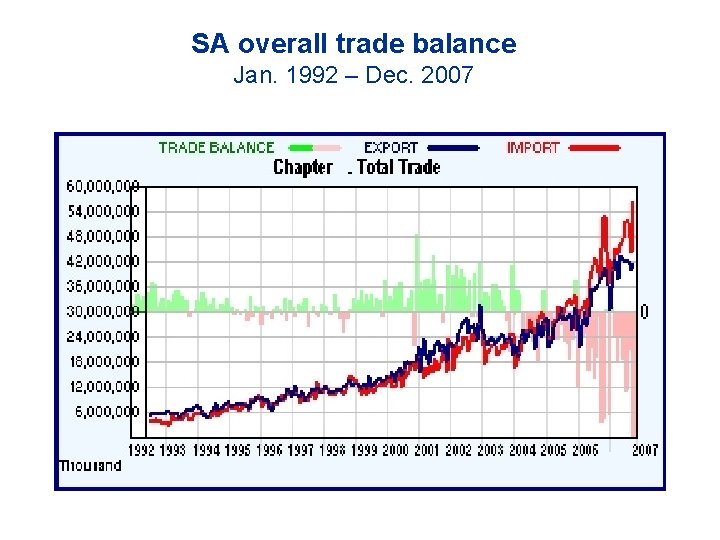
SA overall trade balance Jan. 1992 – Dec. 2007

Imports and exports of pharmaceuticals er armaceutical imports 2006: Tariffs) R 9. 05 billion (US$ 1. 3 Bn), exports R 820 million (US$ 117 million). 2007: Imports R 10. 44 billion, exports R 1. 01 billion NB: This excludes active pharmaceutical ingredients (API’s), which are almost 100% imported. Overall, the trade deficit in the healthcare goods sector (pharmaceuticals incl. API’s, medical devices and medical diagnostics) was over 22 billion Rands in 2007.

Imports and exports of pharmaceuticals Jan. 1992 – Jan. 2008

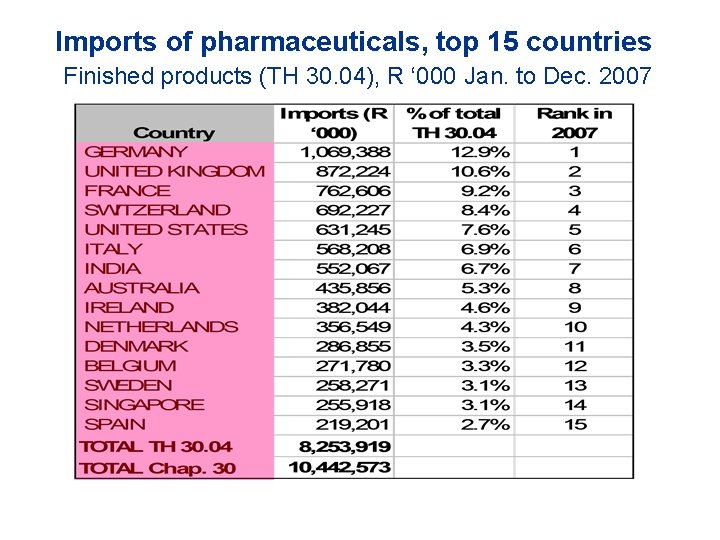
Imports of pharmaceuticals, top 15 countries Finished products (TH 30. 04), R ‘ 000 Jan. to Dec. 2007

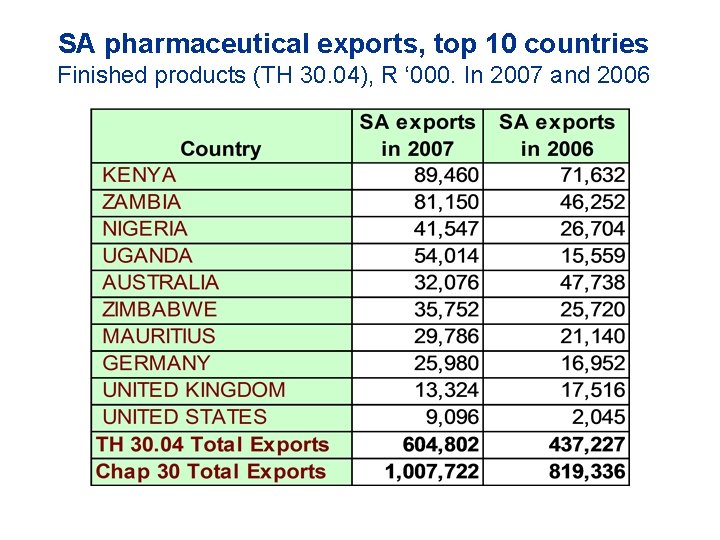
SA pharmaceutical exports, top 10 countries Finished products (TH 30. 04), R ‘ 000. In 2007 and 2006

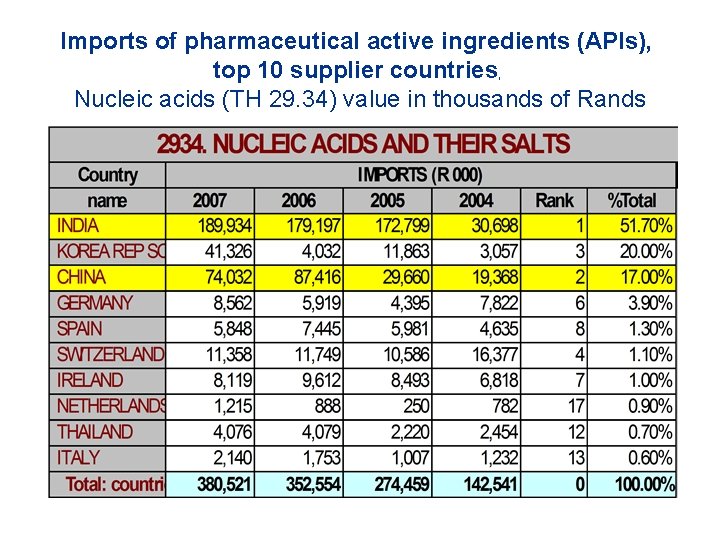
Imports of pharmaceutical active ingredients (APIs), top 10 supplier countries, Nucleic acids (TH 29. 34) value in thousands of Rands

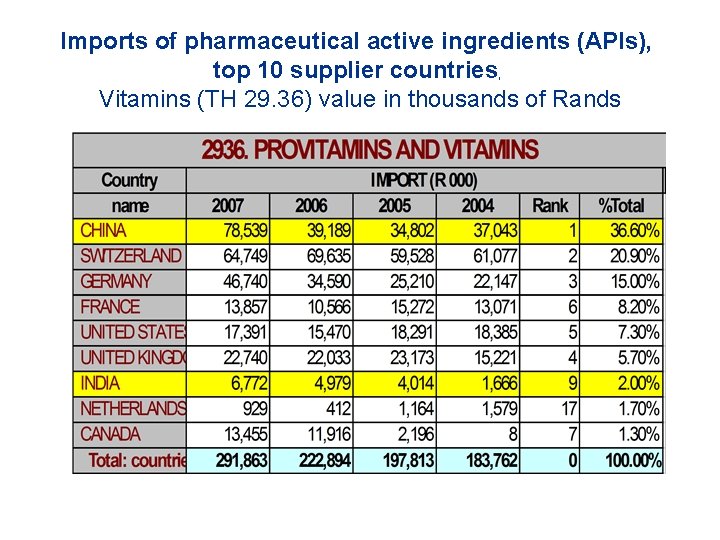
Imports of pharmaceutical active ingredients (APIs), top 10 supplier countries, Vitamins (TH 29. 36) value in thousands of Rands

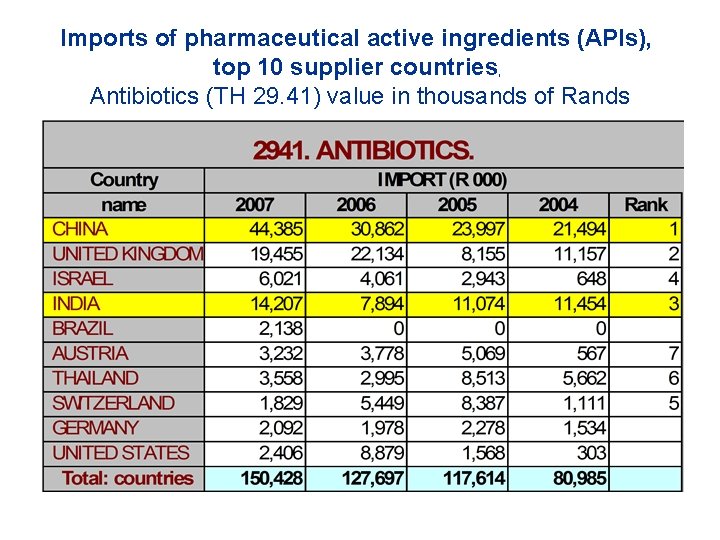
Imports of pharmaceutical active ingredients (APIs), top 10 supplier countries, Antibiotics (TH 29. 41) value in thousands of Rands

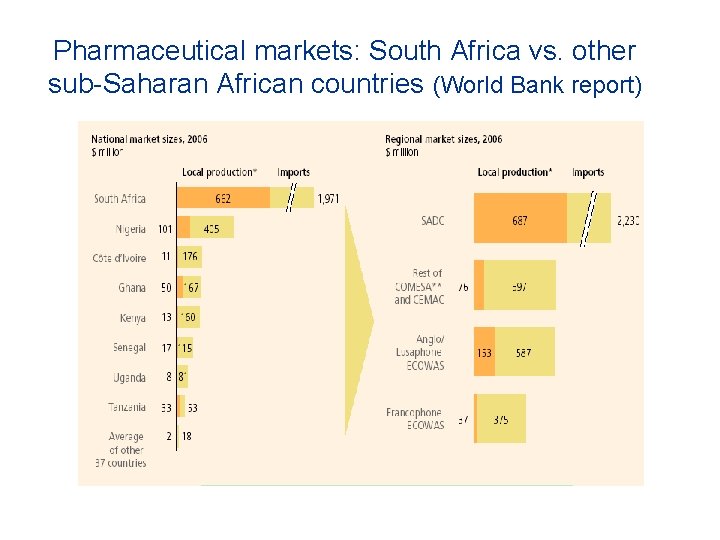
Pharmaceutical markets: South Africa vs. other sub-Saharan African countries (World Bank report)

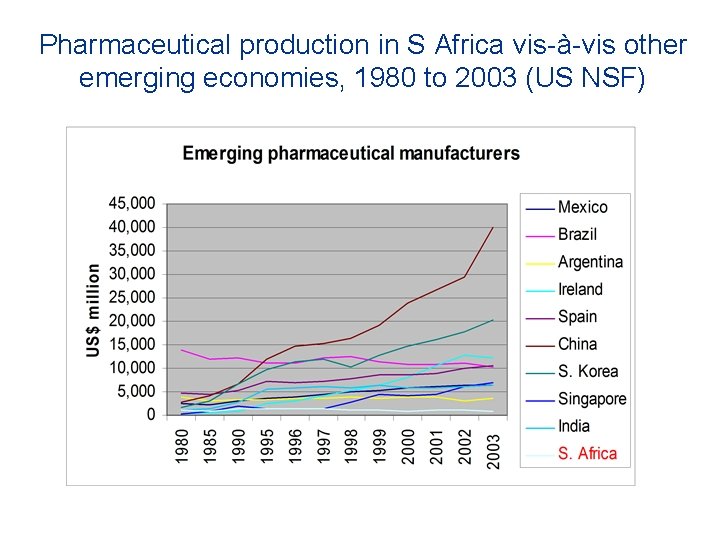
Pharmaceutical production in S Africa vis-à-vis other emerging economies, 1980 to 2003 (US NSF)

Pharmaceutical Sector Strategy: Phase 1 - Pre-feasibility study of domestic manufacture of ARV APIs. Status: Final draft completed, approved by the steering committee (DTI, Do. H & DST), circulated for comments to all associations. Cabinet Memorandum planned for October, Comments and inputs from industry. Phase 2 - Pre-feasibility study, manufacture of anti-TB APIs (in progress); advanced formulation, biologicals….

Pharmaceutical Sector Strategy Key Findings of the ARV API report: Generic API competition is most intense in 1 st generation ARVs, such as Lamivudine, Zidovudine and Nevirapine, which are sold at at a discount relative to the fully inclusive price. Not recommended to compete with these ARVs. There will be 1. 64 million adults on ARVs in SA by 2012 according to Do. H estimates Assuming that 75% of these patients will be on the public. sector’s 1 st line regimen (Efavirenz, Tenofovir and Lamivudine) the requirement for these APIs will be: Efavirenz 325 tons/annum and Tenofovir 135 tpa.

Pharmaceutical Sector Strategy Conclusions of the ARV API report (1) Conclusions of the report, based on the current ARV API market prices, current prices of raw materials and the presently “best in class” manufacturing technology • of Efavirenz and 135 tpa of Tenofovir will cost US$ 72. 5 million (R 564 million @R 7. 8/$), • The non-imported component for ARV APIs will rise from 0% to 40% (presently 100% imported). The local value added will be 30% of the total cost, • The annual revenue of the project will peak at R 2. 6 billion (in 2008 Rands) per annum, • The cost of production of Efavirenz will match or slightly exceed the present market price of $650/kg,

Pharmaceutical Sector Strategy Conclusions of the ARV API report (2) ▪ The cost of production of Tenofovir, including a 5% margin, will slightly exceed the present market price of $730/kg, • Conclusion: Unless there is government support, the project will have a negative NPV of R 520 million and will not be an attractive investment for a private company. Positives: The socio-economic benefits of the project would include: • Local value added R 1. 05 billion • Foreign exchange savings R 1. 7 billion per annum, • Increased security of supply of ARVs, better control over medicine quality, and • Development and diversification of the chemicals sector into high-value fine chemicals’ production; Job creation and skills development.

Pharmaceutical Sector Strategy DTI’s economic incentives (1) • Strategic Industrial Projects (“SIP”) (tax incentive): • SIP started in, phased out in. Recipients of SIP: Roche (Fansidar project) Aspen-Pharmacare (OSD project) On the waiting list: Adcock-Ingram and Aspen-Pharmacare

Pharmaceutical Sector Strategy DTI’s economic incentives (2) • Tax incentives (“SIP-2”) (tax deductions / tax allowances). • Draft Revenue Laws Amendment Bill, 2008, and the “Explanatory Memorandum on the Revenue Laws Amendment Bill” published for comments on the National Treasury website • Comments invited until 5 September 2008 • Tabling in Parliament in late September / early October, launch in January 2009.

Pharmaceutical Sector Strategy DTI’s economic incentives (3) • Tax incentives (“SIP-2”) (tax deductions / tax allowances). Objective: To increase the competitiveness of manufacturing sectors by promoting / incentivising investment in advanced technologies (new products), equipment and skills. ▪ Greenfield investment projects, ▪ Brownfield / expansion investment projects, and ▪ Substantial upgrade projects (recapitalization / replacement of aged / old technology equipment.

Pharmaceutical Sector Strategy DTI’s economic incentives (4) • Types of qualifying projects and limits: Greenfield investment projects: minimum R 200 million in industrial assets (max. R 1. 5 billion). ▪ Brownfield / expansion investment projects: R 30 million or 25% of the value of existing assets. ▪ Substantial upgrade projects (recapitalization / replacement of aged / old technology equipment. R 30 mill or 25% of the value of existing assets. • 2 levels of incentives for: (I) Preferred status projects and (II) Ordinary (qualifying) status projects. ▪

Pharmaceutical Sector Strategy DTI’s economic incentives (5) • Criteria (scoring system) to determine the status of projects: (a) Energy efficiency (index: MWh energy consumed per R 1 million value added); Benchmarked against applicant’s current index (improvement) and industry average, (b) Other environmental considerations (cleaner production etc. ) (c) Technology innovation, new products, (d) SME linkages, (e) Employment creation (jobs per R 1 million capital investment), and (f) Training (training expenditure as % of wage bill). NB: To qualify for the “SIP-2” incentive, the project must score a certain minimum points for (e) + (f). Time-lines: Draft “SIP-2” to be published for comments end August / early September, launch January 2009.

Pharmaceutical Sector Strategy DTI’s economic incentives (6) Enterprise Investment Programme – Manufacturing Investment Programme (EIP – MIP) Time-lines: EIP – MIP Launched 21 July 2008. Will remain available for the next 6 years, until 2014. An investment incentive to stimulate investment growth, in line with the National Industrial Policy Framework (NIPF). Aim: To enhance the sustainability of manufacturing investment projects by SME’s and to support large-to-medium investment projects in manufacturing that would otherwise not be established without the grant. Projects above R 5 million qualify for an investment grant between 15% and 30% of their qualifying investment costs, calculated on a regressive scale, payable over two years. Grant max. R 30 million.

Pharmaceutical Sector Strategy DTI’s economic incentives EIP-MIP (7) The applicant must demonstrate how the EIP - MIP grant is necessary for the project to proceed. Projects are expected to explore other sources of funding before seeking the EIP - MIP grant funding. The principle is to use the EIP - MIP incentive to: Fill funding gaps where there is not sufficient equity for capitalization of the project; Fill funding gaps where cash flows cannot support more third party debt; Influence location of the project in favour of RSA in case where the investor is considering other countries for locating the project Satisfy the company’s internal investment evaluation criteria (IRR or NPV).

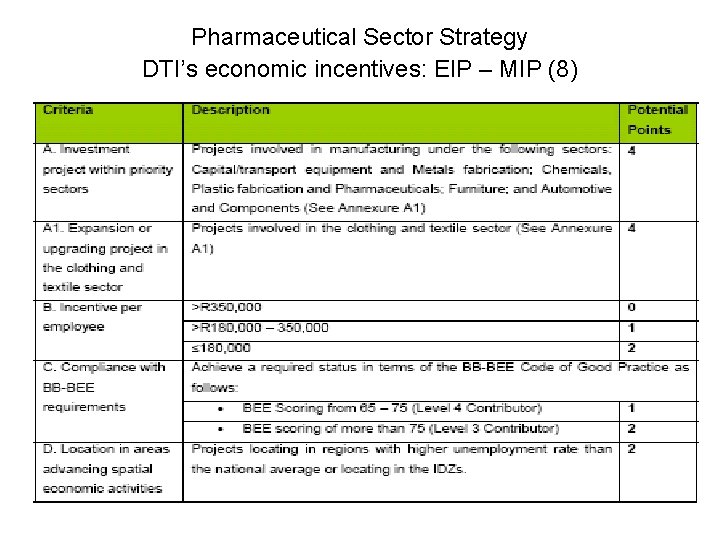
Pharmaceutical Sector Strategy DTI’s economic incentives: EIP – MIP (8)

THE DEPARTMENT OF TRADE AND INDUSTRY www. thedti. gov. za Contact address: Andrek@thedti. gov. za Tel +27 12 3941384; cell +27 83 276 0376
 Biotech due diligence
Biotech due diligence Importance of quality control in pharmaceutical industry
Importance of quality control in pharmaceutical industry Concurrent validation in pharmaceutical industry
Concurrent validation in pharmaceutical industry Fraud in pharmaceutical industry
Fraud in pharmaceutical industry Good manufacturing practices in pharmaceutical industry
Good manufacturing practices in pharmaceutical industry Enterprise risk management pharmaceutical industry
Enterprise risk management pharmaceutical industry Mechanical hazards in industry
Mechanical hazards in industry Rutgers pharmaceutical industry fellowship
Rutgers pharmaceutical industry fellowship Toc in pharmaceutical industry
Toc in pharmaceutical industry Satisfaction mv
Satisfaction mv What is meeting and types of meeting
What is meeting and types of meeting Types of meeting
Types of meeting For todays meeting
For todays meeting Today meeting or today's meeting
Today meeting or today's meeting Sae government industry meeting 2019
Sae government industry meeting 2019 Sae government industry meeting 2019
Sae government industry meeting 2019 Minutes of meeting finance department
Minutes of meeting finance department Math department meeting agenda
Math department meeting agenda Trims and accessories store department in garment industry
Trims and accessories store department in garment industry Department of primary industries and mines
Department of primary industries and mines State of nevada department of business and industry
State of nevada department of business and industry State of nevada department of business and industry
State of nevada department of business and industry Nevada department of business and industry
Nevada department of business and industry Nguyên nhân của sự mỏi cơ sinh 8
Nguyên nhân của sự mỏi cơ sinh 8 Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể độ dài liên kết
độ dài liên kết