MEET THE MOLE The Mole Avogadros hypothesis suggests




- Slides: 11

MEET THE MOLE

The Mole • Avogadro's hypothesis suggests that we can compare the number of molecules in a gas sample. • Even though we can’t see them. A standard number of molecules is called a mole (mol). A mole always contains the same number of molecules. A mole is 6. 02 x 1023 A mole of CO 2 contains the same number of molecules as O 2 molecules

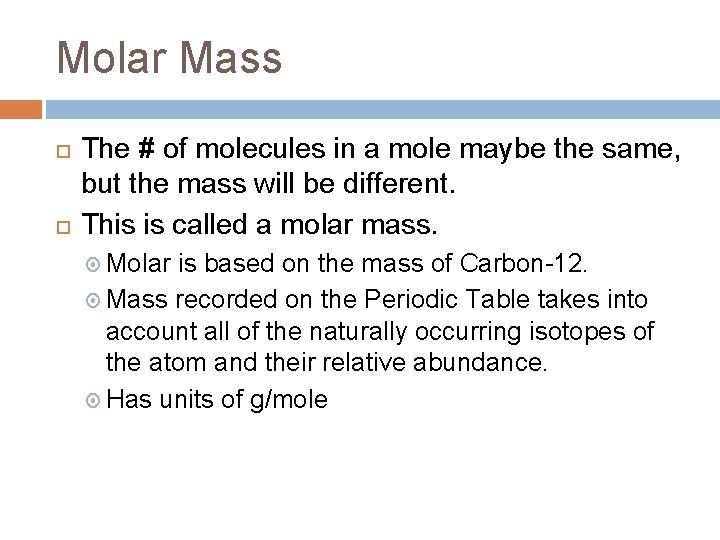
Molar Mass The # of molecules in a mole maybe the same, but the mass will be different. This is called a molar mass. Molar is based on the mass of Carbon-12. Mass recorded on the Periodic Table takes into account all of the naturally occurring isotopes of the atom and their relative abundance. Has units of g/mole

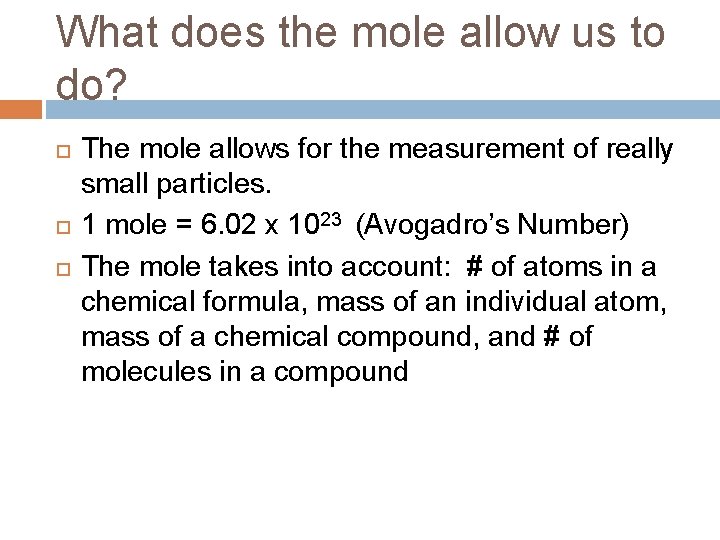
What does the mole allow us to do? The mole allows for the measurement of really small particles. 1 mole = 6. 02 x 1023 (Avogadro’s Number) The mole takes into account: # of atoms in a chemical formula, mass of an individual atom, mass of a chemical compound, and # of molecules in a compound

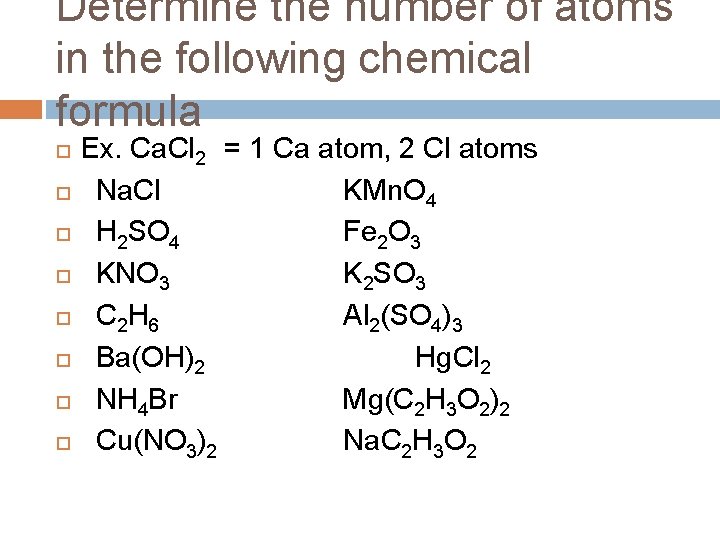
Determine the number of atoms in the following chemical formula Ex. Ca. Cl 2 = 1 Ca atom, 2 Cl atoms Na. Cl KMn. O 4 H 2 SO 4 Fe 2 O 3 KNO 3 K 2 SO 3 C 2 H 6 Al 2(SO 4)3 Ba(OH)2 Hg. Cl 2 NH 4 Br Mg(C 2 H 3 O 2)2 Cu(NO 3)2 Na. C 2 H 3 O 2

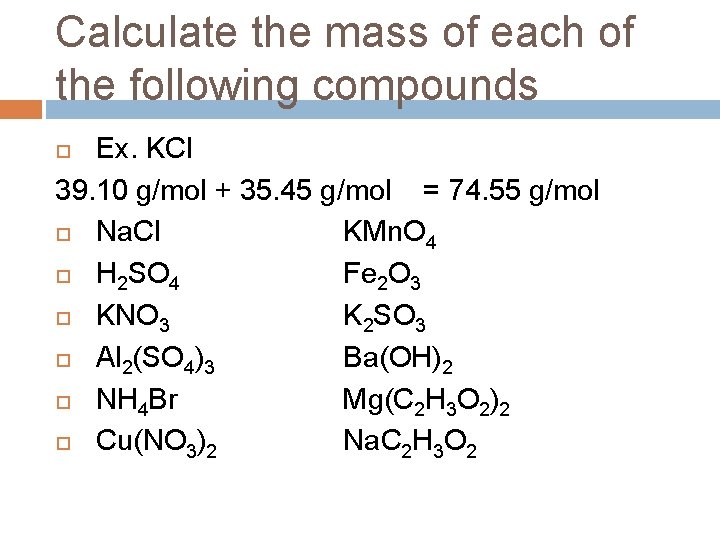
Calculate the mass of each of the following compounds Ex. KCl 39. 10 g/mol + 35. 45 g/mol = 74. 55 g/mol Na. Cl KMn. O 4 H 2 SO 4 Fe 2 O 3 KNO 3 K 2 SO 3 Al 2(SO 4)3 Ba(OH)2 NH 4 Br Mg(C 2 H 3 O 2)2 Cu(NO 3)2 Na. C 2 H 3 O 2

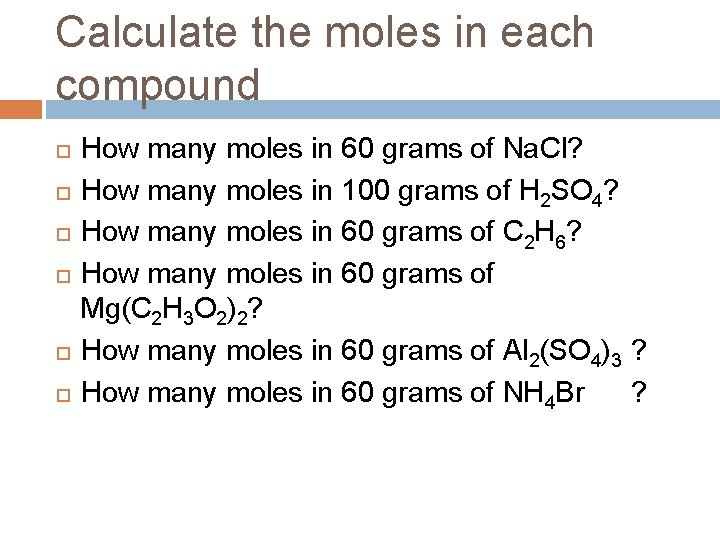
Calculate the moles in each compound How many moles in 60 grams of Na. Cl? How many moles in 100 grams of H 2 SO 4? How many moles in 60 grams of C 2 H 6? How many moles in 60 grams of Mg(C 2 H 3 O 2)2? How many moles in 60 grams of Al 2(SO 4)3 ? How many moles in 60 grams of NH 4 Br ?

Avogadro’s # put to work… Avogadro’s # (1 mole = 6. 02 x 1023 ) is used to convert between moles of a substance and the number of molecules, atoms, particles. One can also convert between mass of a substance and number of molecules, atoms, particles. Convert mass to moles first

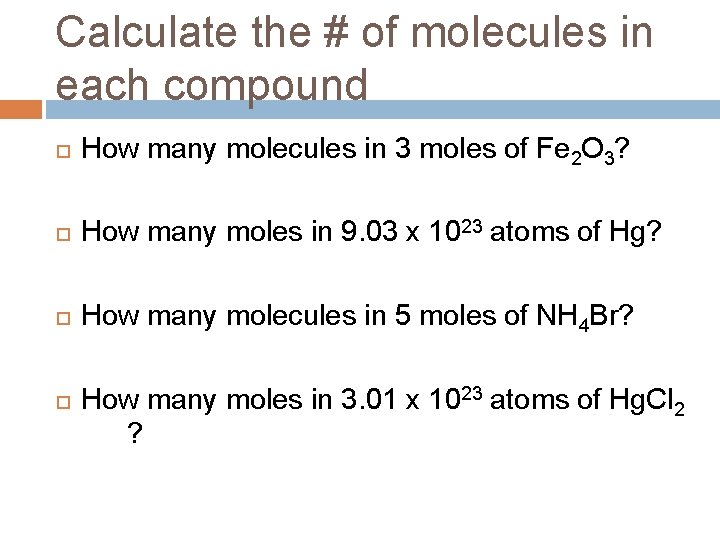
Calculate the # of molecules in each compound How many molecules in 3 moles of Fe 2 O 3? How many moles in 9. 03 x 1023 atoms of Hg? How many molecules in 5 moles of NH 4 Br? How many moles in 3. 01 x 1023 atoms of Hg. Cl 2 ?

Now for some practice… Calculate the molar mass of oxygen

Now for some practice… How many dozen are 60 eggs? How many eggs are in 13 dozen? What is the mass of 2. 50 moles of oxygen? How many moles are in 4. 00 g oxygen? What is the mass of 2. 50 mol carbon dioxide? How many moles are in 4. 00 g carbon dioxide?
 The mole and avogadros number worksheet
The mole and avogadros number worksheet What is avogadros hypothesis
What is avogadros hypothesis The efficient market hypothesis suggests that _______.
The efficient market hypothesis suggests that _______. Projective test definition psychology
Projective test definition psychology God be with you until we meet again
God be with you until we meet again Oxidationstal
Oxidationstal Avogadro's number unit
Avogadro's number unit Avogadro law
Avogadro law What is the symbol of avogadro number
What is the symbol of avogadro number Avogadro's law
Avogadro's law Avogadros principle
Avogadros principle Percent mass formula
Percent mass formula