Medicinal Plants have a far more complex chemistry








































- Slides: 47

Medicinal Plants have a far more complex chemistry than animals, and secondary chemicals from plants have been recognized for their medicinal value for at least 4, 000 years. The first pharmacopoeia, a list of herbal cures, was Chinese, called the Pun-Tsao. Of similar age was the poetry of the Indian Rig-Veda, which included herbal medicines. As a medical practice, it is called Ayurvedic medicine. Only slightly newer was the Aztec collection of medical knowledge, written in the Badianus Manuscript.

More recent, but better known, are the herbal remedies described by Hippocrates around 400 BC and the Greek. Roman military physician Dioscorides. Some of the remedies they described (or derivatives from them) are still in use today: Willow bark tea, which contains salicin (the precursor of acetylsalicylic acid [aspirin]) was prescribed for pain and headache, as well as other gout and other ailments it may not alleviate. By the middle ages, and particularly with the invention of the printing press, a variety of herbals were published with drawings and descriptions of plants useful as herbal remedies, as well as the diseases they were useful in treating.

One of the important early herbalists was Otto Brunfels. He was a true Renaissance man, a priest, later a Protestant, and an accurate observer. He published a number of volumes of herbal medicines, including Onomastikon medicinae, continens omnia nomina herbarum, fruticum etc. (1534).

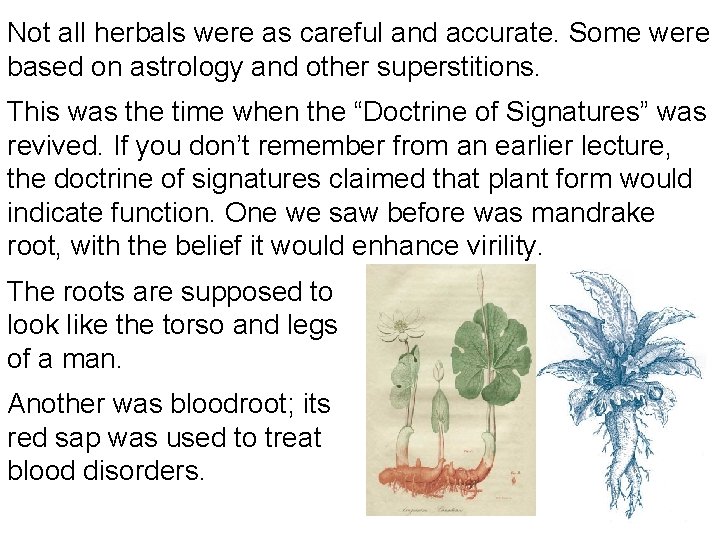
Not all herbals were as careful and accurate. Some were based on astrology and other superstitions. This was the time when the “Doctrine of Signatures” was revived. If you don’t remember from an earlier lecture, the doctrine of signatures claimed that plant form would indicate function. One we saw before was mandrake root, with the belief it would enhance virility. The roots are supposed to look like the torso and legs of a man. Another was bloodroot; its red sap was used to treat blood disorders.

From the opposite perspective, a significant amount of the descriptive botany prior to Linnaeus came from the work of physicians, and remember that Linnaeus was a physician. It was those physicians who described remedies using herbals that led to many modern treatments. They developed extracts from effective plants, and those extracts are (though many are now synthesized, rather than extracted) the basis of modern treatments. Example 1: William Withering made the association between a folk remedy of foxglove and its success in treating congestive heart failure in 1775. He extracted digitalis (the name from the plant species, Digitalis purpurea) and established its use.

Digitalis contains a number of cardiac glycosides, among which is digoxin, which is the modern drug administered. As a drug it increases the strength of cardiac contraction and tends to prevent arrythmia. However, in excess it is toxic; it causes nausea, anorexia and vomiting. It has, as a result, been sometimes abused as a weight loss drug. William Withering is considered to be the “father” of modern pharmaceutical chemistry.

Example 2: Friedrich Serturner isolated morphine from the opium poppy in 1805. He named the drug for the Greek god of dreams, Morpheus. This sap contains the morphine. In 1874 a German chemist discovered that adding acetyl groups caused the drug to dissolve in the fatty layers of the brain far more rapidly, and thus bind more readily to the opiate receptors. Binding leads to the physiological responses. Those receptors also bind endorphins that our bodies synthesize.

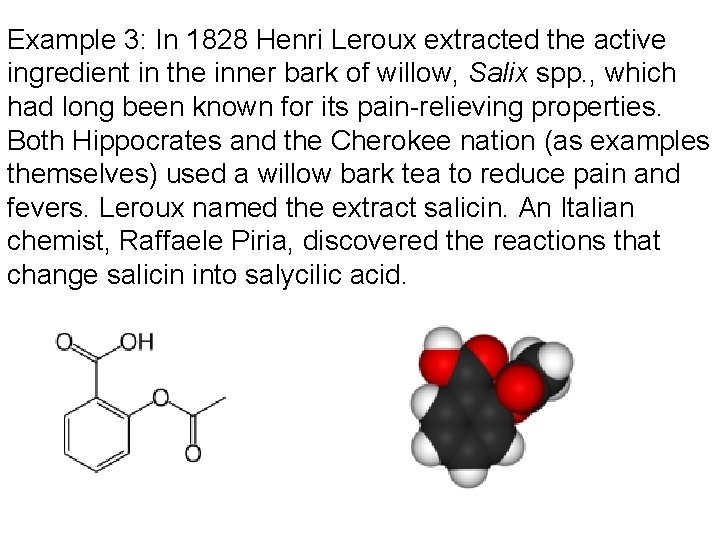
Example 3: In 1828 Henri Leroux extracted the active ingredient in the inner bark of willow, Salix spp. , which had long been known for its pain-relieving properties. Both Hippocrates and the Cherokee nation (as examples themselves) used a willow bark tea to reduce pain and fevers. Leroux named the extract salicin. An Italian chemist, Raffaele Piria, discovered the reactions that change salicin into salycilic acid.

Salicylic acid irritates the stomach. In 1853 Charles Gerhardt neutralized the acidity (buffering) by turning salycilic acid into acetylsalycilic acid. Gerhardt was not interested in manufacturing. In 1897 a chemist named Hoffman at Bayer, a German drug company, rediscovered Gerhardt’s work. In 1899 Bayer registered the trademark name aspirin (a – for the acetyl groups added, spir – for the plant genus Spiraea, a meadowsweet, from which first extracts were made, or possibly for Piria, and the in – a common ending for drug names. It was the same Hoffman who developed heroin from morphine only 11 days after (re)-discovering aspirin.

It is important to recognize that aspirin, though it began with a plant extract, was the first synthetic drug, a product of “modern chemistry”. It remains the most widely used synthetic drug: • In long term daily use (low dose), it is believed to prevent or reduce the probability of heart attack • it stimulates the immune system • it suppresses prostaglandins and thromboxanes, reducing pain, inflammation and fever • it delays cataract formation However, in children aspirin can interact with chicken pox, leading to a serious disease – Reyes syndrome. The advice is to use acetaminophen (Tylenol) for kids.

There are many more examples, but before looking at some it’s important to recognize how large a fraction of our medicines either are still extracted from plants or are now synthesized, but were originally derived from plants. At least 25% of all prescriptions have active ingredients that are derived from plants. At least a similar fraction contain active ingredients synthesized based on plant extracts. If we (somewhat loosely) include ingredients derived from fungi (e. g. penicillin and the other –cillin antibiotics), the fraction is far larger than 50%. In addition, there is a huge market for herbal ‘supplements’ - ~$20 billion dollars/year.

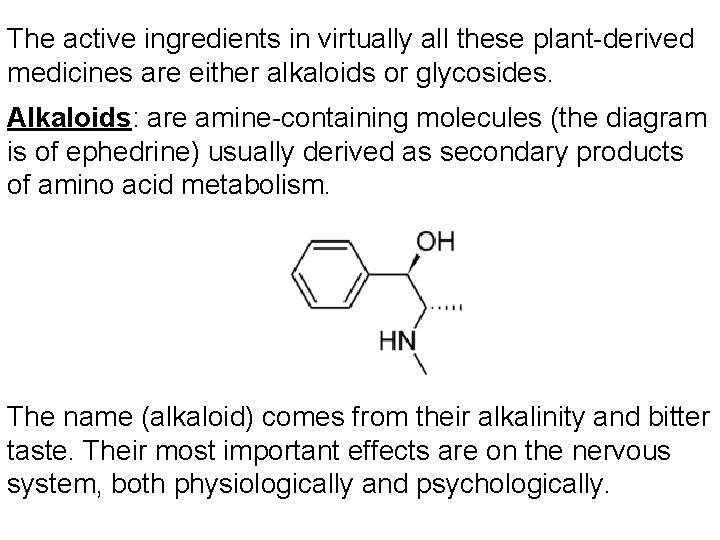
The active ingredients in virtually all these plant-derived medicines are either alkaloids or glycosides. Alkaloids: are amine-containing molecules (the diagram is of ephedrine) usually derived as secondary products of amino acid metabolism. The name (alkaloid) comes from their alkalinity and bitter taste. Their most important effects are on the nervous system, both physiologically and psychologically.

Animals and fungi also may produce alkaloids. Important examples include caffeine, nicotine, morphine, cocaine, quinine, atropine, scopolamine, mescaline, theobromine, vincristine, vinblastine and ephedrine. Note the –ine ending on al these names. We’ll return to some of these in considering more examples of medicines A few don’t end in –ine: psilocybin, lysergic acid Others are poisonous, e. g. strychnine and coniine.

Glycosides: get their collective name because the active ingredient is attached to a sugar (-glyco). There are 3 main categories: cyanogenic glycosides, cardiac glycosides and saponins. Cyanogenic glycosides release HCN when the linkage is cleaved by digestive enzymes. HCN is toxic, therefore, in general these glycosides have no medical application. There is one controversial exception – laetrile, a cancer treatment derived from peach pits, in which the HCN is supposedly released only within cancerous cells.

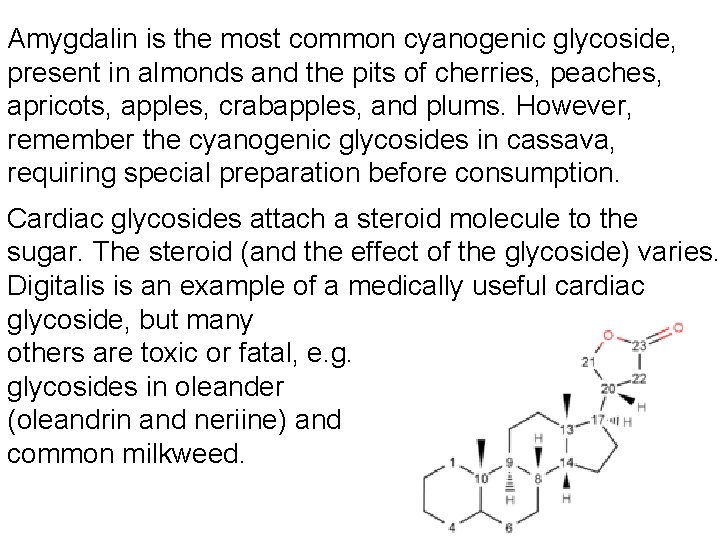
Amygdalin is the most common cyanogenic glycoside, present in almonds and the pits of cherries, peaches, apricots, apples, crabapples, and plums. However, remember the cyanogenic glycosides in cassava, requiring special preparation before consumption. Cardiac glycosides attach a steroid molecule to the sugar. The steroid (and the effect of the glycoside) varies. Digitalis is an example of a medically useful cardiac glycoside, but many others are toxic or fatal, e. g. glycosides in oleander (oleandrin and neriine) and common milkweed.

These chemicals are generally believed to have evolved as plant defenses against herbivores. A cardiac glycoside is the best example of turning this around. The glycoside in milkweed sap is taken up and sequestered in sacs along the sides of the Monarch butterfly caterpillar. It does not poison the caterpillar, who then uses it as a defense against predators who might eat it.

Within a very few minutes of eating a butterfly (or caterpillar) raised on milkweed, the bluejay upchucks.

Saponin glycosides are mostly harmful, since they cause red blood cell lysis. However, in low doses they are used as expectorants (break up phlegm and make it possible for you to cough it out). Liquorice contains a saponin glycoside. The name derives from their similarity in action to soaps, and saponins are sometimes used as mild detergents. That also explains how they cause cell lysis – by acting like a detergent breaking up ‘fatty’ cell membranes. Also, remember that the part attached to the sugar is a steroid. Diosgenin from Dioscorea (true yam) was the plant source for the first birth control pills.

There a number of other glycosides that don’t fit into the 3 groups that are of interest: Arbutin – from common Bearberry (Arctostaphylos uvaursi) – known to Inuit and northern native cultures as a urinary antiseptic Steviol glycosides – from Stevia rebaudiana bertoni – are natural sweeteners used in many cultures that are 40 – 300 x ‘sweeter’ than sucrose

Anthraquinone glycosides – from Aloe vera – effectively reduce pain, enhance healing, particularly from 1 st degree burns. This use dates back to ancient Egypt Sinigrin and sinalbin – thiol (sulfur containing) glycosides that are the flavor of black and white mustard

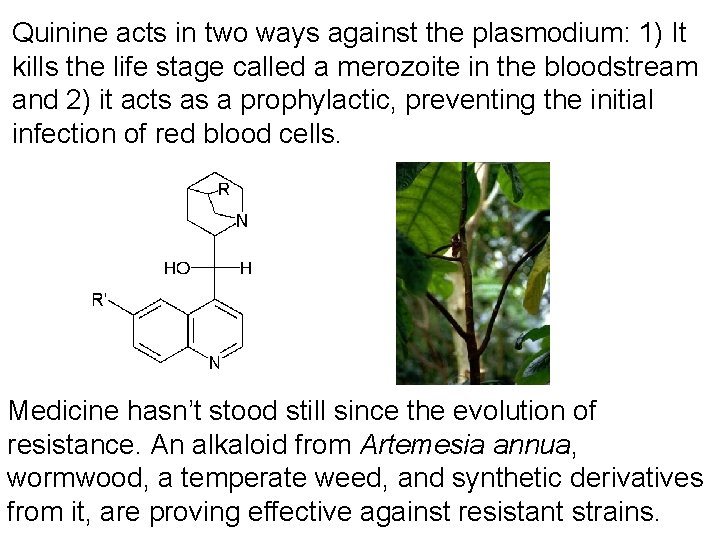
Now let’s go back to the alkaloids, and consider some examples from the enormous variety… 1. Quinine – an alkaloid initially derived from a South American genus, Cinchona. It is in the same family as coffee. The Incas knew of its herbal benefits; they apparently treated the wife of the Viceroy, the Countess of Cinchon. Her recovery from the symptoms of malaria led Linneaus to name the plant after her. The bark of Cinchona was powdered to make the treatment. During the 17 th and 18 th centuries the bark became quite valuable and expensive. In 1820 Pierre Pelletier and Joseph Cavetou isolated quinine from the bark powder, and soon quinine replaced bark powder in treatment.

However, large quantities were needed for extraction. Plantations were established in India and Java, and a high yield variety of Cinchona ledgeriana was developed in Bolivia. A synthetic drug with fewer side effects, chloroquine, was developed in 1944 by American chemists Woodward and Doering. Chloroquine replaced quinine as the treatment of choice. Recently there has been a return to quinine treatment because the organism most frequently responsible for malaria, Plasmodium falciparum, has become resistant to chloroquine. R. B. Woodward Nobel prize 1965 W. Doering

Quinine acts in two ways against the plasmodium: 1) It kills the life stage called a merozoite in the bloodstream and 2) it acts as a prophylactic, preventing the initial infection of red blood cells. Medicine hasn’t stood still since the evolution of resistance. An alkaloid from Artemesia annua, wormwood, a temperate weed, and synthetic derivatives from it, are proving effective against resistant strains.

2. Treatment of schizophrenia using drugs has drawn from 4000 year old Indian herbology to find snakeroot, Rauwolfia serpentina, and an alkaloid within called reserpine. This drug was only isolated from snakeroots in 1952. It is an antipsychotic and antihypertensive. It works by depleting monoamine neurotransmitters (dopamine, norepinephrine, and serotonin). Due to various side effects (nausea, vomiting, gastric ulceration, depression), its use as an antipsychotic has effectively ended. It is still used as an antihypertensive. Even where it is not used, many other alkaloids used are also derived from snakeroot.

3. Ephedrine is an alkaloid used in herbal medicine as a decongestant. It is extracted from species in the genus Ephedra. That use reaches back thousands of years in China, where the regional species, Ephedra sinica, has its own common name, Ma Huang. However, ephedrine is not used as a decongestant much in North America. Instead, it is used as a metabolic stimulant, in weight loss drugs, and in body sculpting. It is banned by the IOC and NCAA.

Why? Its physiological effects are similar to those of amphetamines. Amphetamine and methamphetamine are synthetic derivatives of ephedrine. It “turns on” thermogenesis, the burning of calories (largely fat) to produce heat, stimulates the heart (heart rate and blood pressure), relaxes bronchial muscles permitting greater air intake, and increases blood flow to muscles and brain. That explains why it is banned, but you need to know it is also dangerous. Its use can lead to (among many effects) tachycardia, cardiac arrythmia, hypertension, delusion and paranoia. In various over-the-counter and prescription medicines, pseudoephedrine has replaced ephedrine with fewer side effects, though it, too, is banned in sports.

4. Therapeutic alkaloids in cancer treatment - blue periwinkle, Catharanthus roseus, from Madagascar contains alkaloids vinblastine and vincristine which are very effective against leukemia and lymphoma. vincristine Vincristine works by binding to tubulin, a protein critical in microtubule structure that, among other functions, makes up the mitotic spindle. The cell division that characterizes cancer is prevented.

The concentrations of these alkaloids is very low. Your text tells you it takes 53 tons of leaves to make 100 g of vincristine. Vincristine is (carefully) injected intravenously in the treatment of childhood leukemia, Hodgkin’s lymphoma, and non-Hodgkin’s lymphoma as part of differing chemotherapy regimes for each disease. Vinblastine is used to treat both Hodgkin’s and non. Hodgkin’s lymphomas. Both vinca alkaloids were isolated by Robert Noble and Charles Beer from periwinkle. There are many other potent alkaloids in this species, though none are in wide therapeutic use yet.

5. Taxol (paclitaxel) is yet another example of a drug found ‘just in time’. Taxol is extracted from the bark of the Pacific yew, Taxus brevifolia. The Pacific yew grows naturally in the old growth forests of the Pacific northwest of the U. S. , British Columbia and northward into southern Alaska. Lumber companies harvesting ‘desirable’ trees from these forests considered the yew to be a ‘junk’ tree, and cut them only to clear cut areas for re-planting. The drug yield is very small, and pacific yew grows very slowly. It requires the yield of 6 100 -year old trees to treat one patient. The yew was on the edge of becoming endangered. What to do?

The drug was spotted in yew bark in 1963 and only isolated from yew bark in 1967 (by Monroe Wall and Mansukh Wani) from samples of 30, 000 plants (in the case of the yew, needles, twigs, and bark) provided to scan for anti-tumor activity in mice. Taxol has a complicated structure, and lab synthesis is still not possible. However, a number of alternate means of production have been developed.

1. Taxol can be “semi-synthesized” using a much more abundant chemical found in a number of other yews. Cell culture has also been used to produce that starting chemical, deacetylbaccatin. 2. Fermentation (combined with a little genetic engineering) technology can be used to produce taxol -like chemicals from actinobacteria, or paclitaxel itself from Nodulisporium sylviforme culture. 3. Plant cell fermentation is now used to produce most taxol using a specific callus tissue from Taxus. The drug is extracted from the culture, separated by chromatography, and purified by crystallization.

Taxol works in a way opposite to other anti-cancer drugs mentioned thus far. Rather than preventing tubulin organization into the spindle apparatus, taxol binds to microtubules and ‘hyper’stabilizes their structure. You can think of this as rigidly fixing the structure. Mitosis can’t be completed (microtubules can’t disassemble), nor a new cycle begun after that binding. Taxol also causes cell death (apoptosis) by binding to and blocking the activity of a protein, Bcl-2 (B cell leukemia 2). That protein blocks apoptosis. Paclitaxel is an effective treatment for lung, ovarian, and breast cancer, and advanced forms of Karposi’s sarcoma. A related taxoid drug, Taxotere, is now used to treat drug-resistant and metastatic breast cancer.

6. A tree relative of tupelo, Camptotheca acuminata, was the original source for a drug used to treat leukemia and cancers of the liver and stomach in China as an herbal therapy. The drug was/is camptothecin, an alkaloid secondary chemical. Higher concentrations of the same drug were found in the stem wood of Nothopodytes foetida from western India. It was Dr. Monroe Wall who ‘discovered’ the anti-cancer properties of the tree’s bark and wood in 1958, and, with others, isolated the drug from plant material in 1966. It was tried on mice and on patients with gastro-intestinal cancers in the early 1970’s, then stopped due to severe side effects.

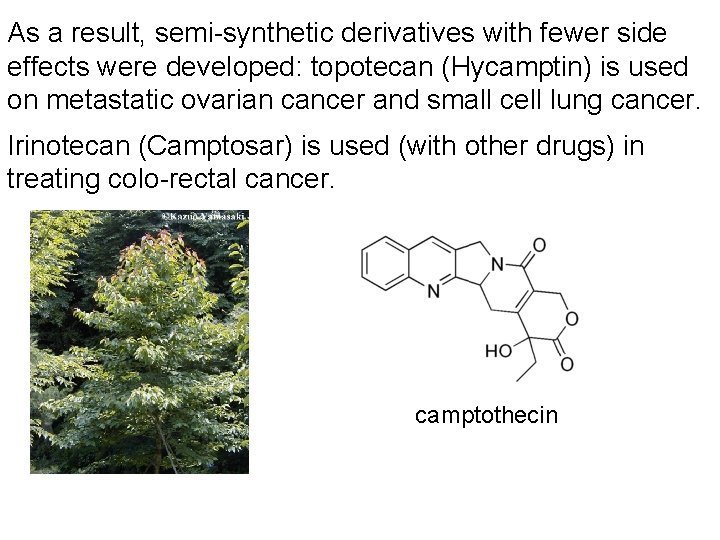
As a result, semi-synthetic derivatives with fewer side effects were developed: topotecan (Hycamptin) is used on metastatic ovarian cancer and small cell lung cancer. Irinotecan (Camptosar) is used (with other drugs) in treating colo-rectal cancer. camptothecin

Camptothecin and its derivatives work differently. They affect the function of an enzyme, topoisomerase I. This enzyme normally cleaves DNA, unwinds it, then religates the DNA it cleaved. Once camptothecin is bound to the enzyme, it can cleave but not re-ligate DNA. As a result, there are numerous single strand breaks in the DNA, and dividing cells can’t properly complete the process. There are many other anti-cancer drugs derived from plant and bacterial sources. A complete review would take more than an entire semester.

Herbal remedies are used in many other situations. Your text has a short list in table 19. 2. It also notes a caution: licensing and extensive testing are required for prescription and over-the-counter drugs, but as long as an herbal is offered as a dietary supplement, all that is required is that the product be safe, not necessarily effective. It is on that basis that ephedrine-containing and comfreybased products were banned, and warnings required for some others. We’ll concentrate on herbal products that seem to have a proven usefulness…

St. John’s Wort, Hypericum perforatum, is now believed to be a useful herbal remedy for depression. It is also called Klamath weed, and is an invasive, poisonous plant that causes photosensitivity when consumed by some animals. It is ‘controlled’, in an excellent example of biological control, by a beetle, Chrysolina quadrigemina, that feeds on its leaves. Simple physical removal doesn’t work well because it is rhizomatous. How you can recognize it – black dots on the leaves. Chrysolina quadrigemina

St. John’s wort has been used as an herbal medicine since Greek times. Both the Greeks and Native Americans used St. John’s wort for inducing abortion and externally as an anti-inflammatory and antiseptic. Today its use as an antidepressant seems to show significant clinical effect against mild to moderate depression, but to be ineffective against more severe depression. It also apparently has fewer and less severe side effects than many of the other medications used. It may work in the same way as a number of commercial medications as an SSRI (selective serotonin reuptake inhibitor). If that is true, the likely active ingredient is hyperforin.

Ginkgo biloba is an ancient tree usually placed with the Coniferophyta, but sometimes off on its own in a group called the Ginkgophyta. It’s really alone, as there is only one species in the group. It is, without question, a gymnosperm; its seeds are not protected within an ovarian wall, as are the Angiosperms.

The Ginkgo is an ancient plant, with leaf fossils at least 270 million years old. After the Cretaceous it slowly dwindled in abundance and distribution, until today the only native distribution left is in central China. Ginkgo has, however, been grown in many other places. It is a dioecious species, and there is a fairly common male cultivar called “Autumn Gold” that is widely grown (even locally). In China, an herbal tea of ginkgo has been used for 4, 000 years to treat breathing problems (asthma, bronchitis). In modern western medicine, flavenoids in ginkgo extracts seem to have circulatory (improved microcirculation) and nootropic effects (enhances memory and brain function), as well as anti-oxidant function.

The disease against which ginkgo shows promise is Alzheimer’s dementia (along with other mild forms of dementia). It works when used as a daily dietary supplement, but you shouldn’t bother now. It seems to have no material effect on young, healthy adults. However, improved circulation also comes with a negative side effect – it blocks PAF (platelet agregation factor) and slows or prevents clotting. As a result, it is contra-indicated for those who are taking aspirin as part of a heart/circulation regime, pregnant women, and those taking MAO (monoamine oxidase) inhibitors.

One last herbal, of personal interest to males at my age – saw palmetto, Serenoa repens, as a dietary supplement. Saw palmetto is a small palm, growing to no more than 2 – 4 m. Its leaves lead to a secondary description as a ‘fan palm’. It is native to the southeastern U. S.

An extract of the fruit of saw palmetto is suggested to reduce a problem called BPH, benign prostate hyperplasia. The problem is very common, affecting 50% of men over 50 and 90% of men over 70. When the prostate enlarges, it slowly squeezes on the urethra, and makes urination more difficult. The cause of the hyperplasia is dihydrotestosterone, a product of testosterone metabolism (it is also involved in producing male pattern baldness). The fruit extract of saw palmetto inhibits 5 - -reductase, an enzyme that converts testosterone to dihydrotestosterone, and interferes with the binding of DHT to androgen receptors in the prostate.

Saw palmetto extract has fewer side effects and works for a larger percentage of sufferers than the usual, synthetic medicine prescribed. Since it is an herbal medicine, there is controversy about the evidence that point to its usefulness. A few other herbal/medicinal plants and their suggested uses: Echinacea purpurea – purple coneflower, a prairie plant; suggested as an immune system stimulant that shortens the length of the common cold

Valerian, from Valeriana offinalis, is an herbal sedative and apparently useful against insomnia. It is native to Europe and Asia, but has been introduced into North America. Its mode of action is probably related to interaction with GABA ( amino butyric acid), but the mechanism is unknown. Extended use can lead to addiction and withdrawal symptoms.

Ginseng (Panax spp. ) is suggested to stimulate the immune system, increase resistance to stress, and to increase libido and sexual performance. Parts of that may be supported by evidence of anti-oxidant activity; parts are controversial. The active ingredients are gensenosides (triterpene saponins). Siberian ginseng is widely sold as an herbal; it is not a Panax, rather it is Eleutherococcus senticosus, and instead of gensenosides it contains eleutherosides.

In Asia they want American ginseng (P. quinquefolius), and the wild plants have more of the desired chemical. However, the wild plant is becoming very rare. Korean beliefs are that American ginseng promotes ‘Eum’ energy (female), while Asian ginseng (red) promotes ‘Yang’ (male). In North America, it seems Siberian ginseng sells better than North American (Is this an example of “the grass is always greener…”? ) Most of the North American ginseng is produced in Ontario, British Columbia, and Wisconsin.