Medicated Feeds Overview Dr Dragan Momcilovic Medicated Feeds


























- Slides: 50

Medicated Feeds Overview Dr. Dragan Momcilovic, Medicated Feeds Specialist Dr. Gabriel Davila, Staff Fellow Food and Drug Administration Center for Veterinary Medicine Division of Animal Feeds Medicated Feeds Team Rockville, MD June 2012

Agenda n Definitions and usages n n Pre-approval activities n n n FDA’s new animal drug approval process (basics) – Label review Labeling of medicated products Post-approval activities n n Drug Categories Types of Distribution Medicated Products Current Good Manufacturing Practices (c. GMP) Program Monitoring Licensing and Registration Where to look for more information

Drug Categories

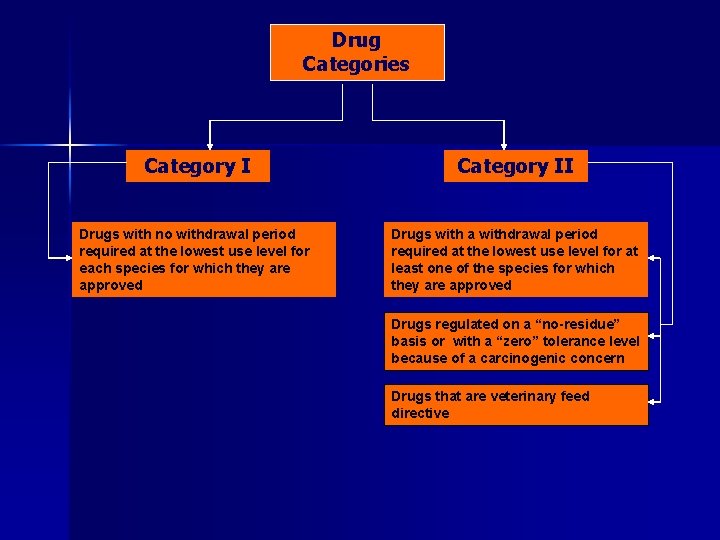
Drug Categories Category II

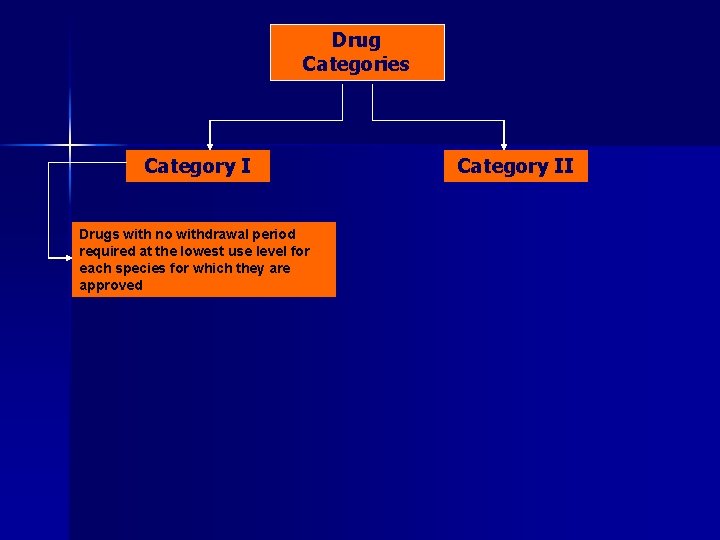
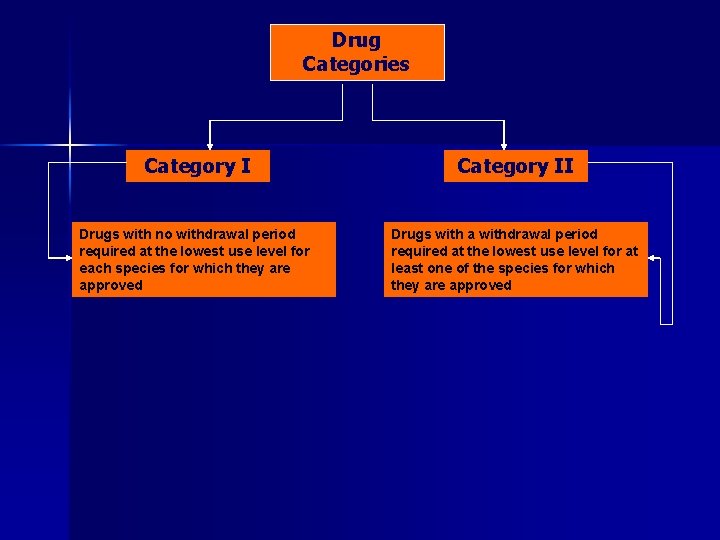
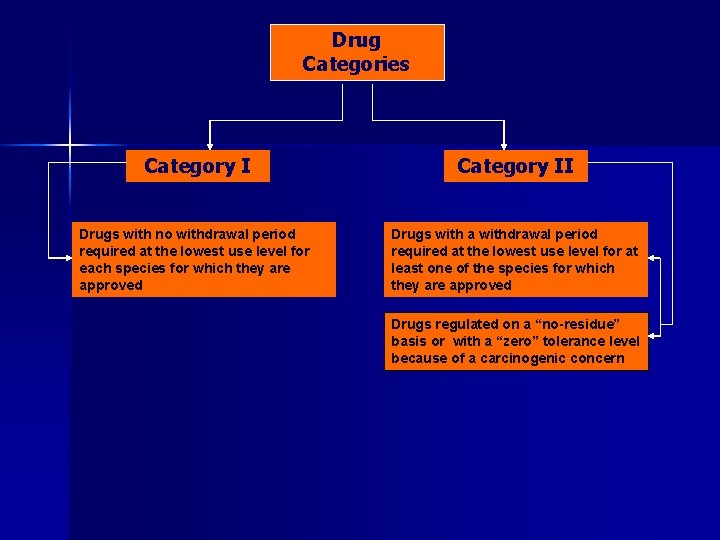
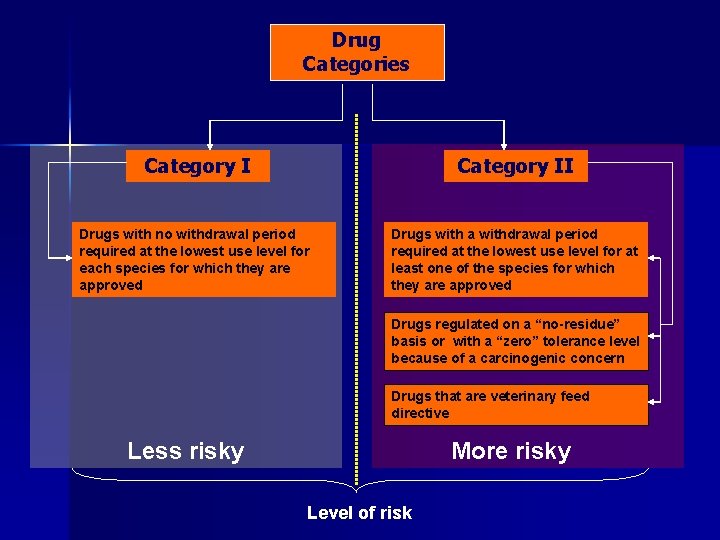
Drug Categories Category I Drugs with no withdrawal period required at the lowest use level for each species for which they are approved Category II

Drug Categories Category II Drugs with no withdrawal period required at the lowest use level for each species for which they are approved Drugs with a withdrawal period required at the lowest use level for at least one of the species for which they are approved

Drug Categories Category II Drugs with no withdrawal period required at the lowest use level for each species for which they are approved Drugs with a withdrawal period required at the lowest use level for at least one of the species for which they are approved Drugs regulated on a “no-residue” basis or with a “zero” tolerance level because of a carcinogenic concern

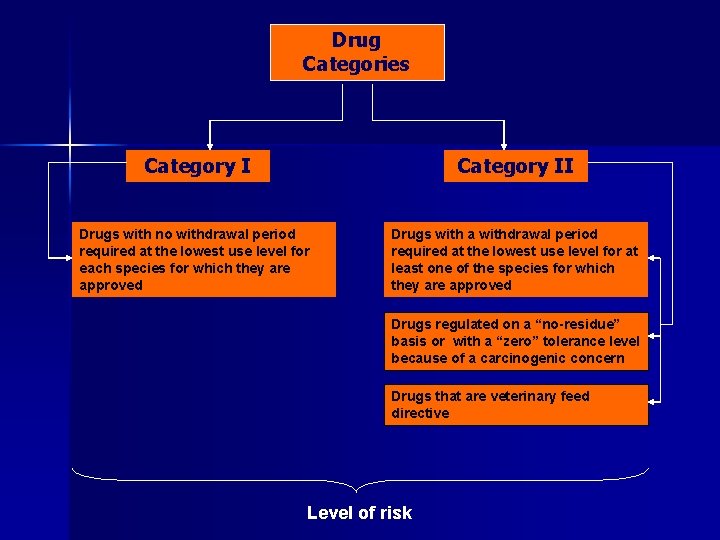
Drug Categories Category II Drugs with no withdrawal period required at the lowest use level for each species for which they are approved Drugs with a withdrawal period required at the lowest use level for at least one of the species for which they are approved Drugs regulated on a “no-residue” basis or with a “zero” tolerance level because of a carcinogenic concern Drugs that are veterinary feed directive

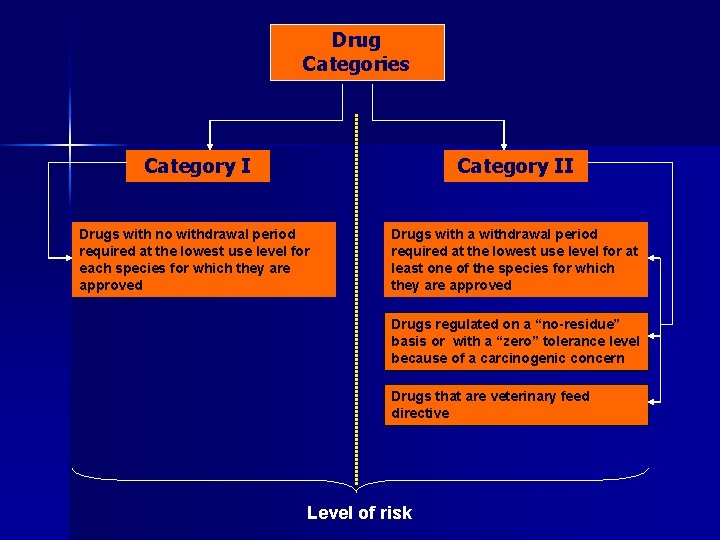
Drug Categories Category II Drugs with no withdrawal period required at the lowest use level for each species for which they are approved Drugs with a withdrawal period required at the lowest use level for at least one of the species for which they are approved Drugs regulated on a “no-residue” basis or with a “zero” tolerance level because of a carcinogenic concern Drugs that are veterinary feed directive Level of risk

Drug Categories Category II Drugs with no withdrawal period required at the lowest use level for each species for which they are approved Drugs with a withdrawal period required at the lowest use level for at least one of the species for which they are approved Drugs regulated on a “no-residue” basis or with a “zero” tolerance level because of a carcinogenic concern Drugs that are veterinary feed directive Level of risk

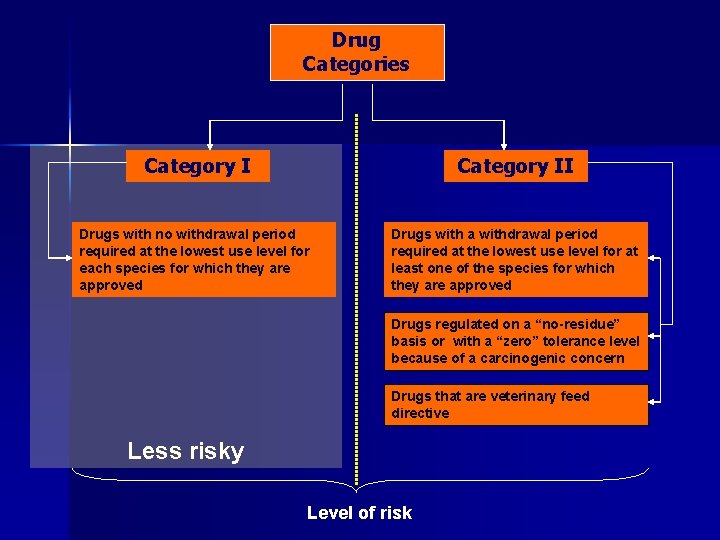
Drug Categories Category II Drugs with no withdrawal period required at the lowest use level for each species for which they are approved Drugs with a withdrawal period required at the lowest use level for at least one of the species for which they are approved Drugs regulated on a “no-residue” basis or with a “zero” tolerance level because of a carcinogenic concern Drugs that are veterinary feed directive Less risky Level of risk

Drug Categories Category II Drugs with no withdrawal period required at the lowest use level for each species for which they are approved Drugs with a withdrawal period required at the lowest use level for at least one of the species for which they are approved Drugs regulated on a “no-residue” basis or with a “zero” tolerance level because of a carcinogenic concern Drugs that are veterinary feed directive Less risky More risky Level of risk

Types of Distribution Medicated Feeds are distributed: - Over-The-Counter - Veterinary Feed Directive

Medicated Products Type A medicated article Type B medicated feed Type C medicated feed

Medicated Products Type A medicated article is a new animal drug

A new animal drug is…. Section 201(v)*: - any drug intended for use for animals other than man, including any drug intended for use in animal feed - does not include animal feed *Federal Food, Drug, and Cosmetic Act

Medicated Products Type A medicated article is a new animal drug with or without inactive ingredients intended for use in animal feed intended solely for further manufacture

Medicated Products Type A medicated article is used to make another Type A medicated article a Type B medicated feed a Type C medicated feed

Animal feed is. . . n Section 201(w)*: – an article intended for use for food for animals other than man – intended for use as a substantial source of nutrients in the diet of the animal – is not limited to a mixture intended to be the sole ration of the animal. *Federal Food, Drug, and Cosmetic Act

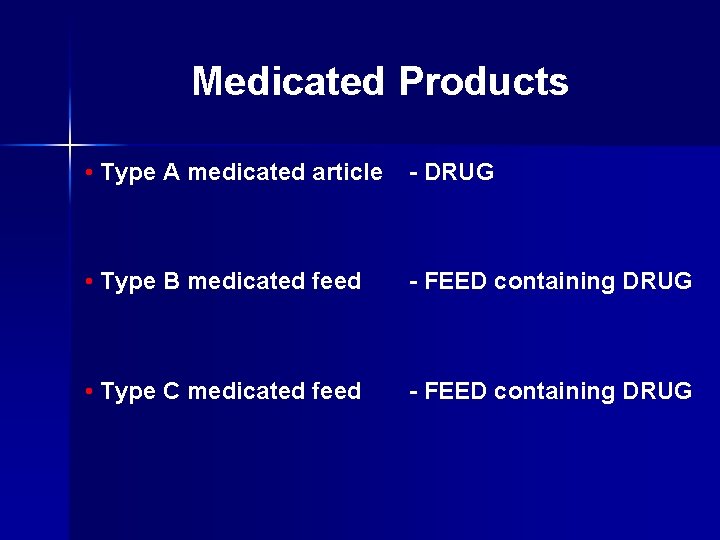
Medicated Products • Type A medicated article - DRUG • Type B medicated feed - FEED containing DRUG • Type C medicated feed - FEED containing DRUG

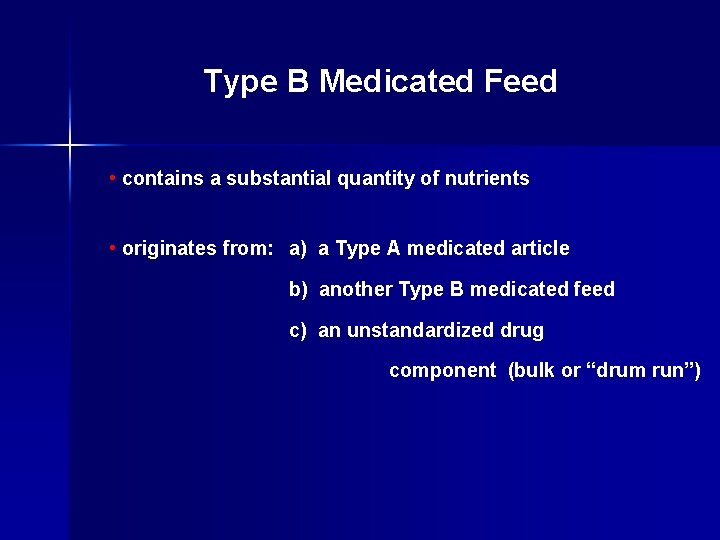
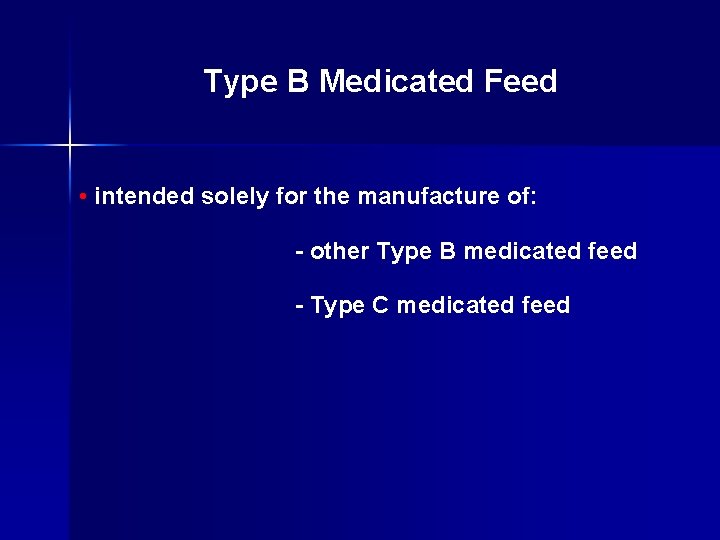
Type B Medicated Feed • contains a substantial quantity of nutrients • originates from: a) a Type A medicated article b) another Type B medicated feed c) an unstandardized drug component (bulk or “drum run”)

Type B Medicated Feed • intended solely for the manufacture of: - other Type B medicated feed - Type C medicated feed

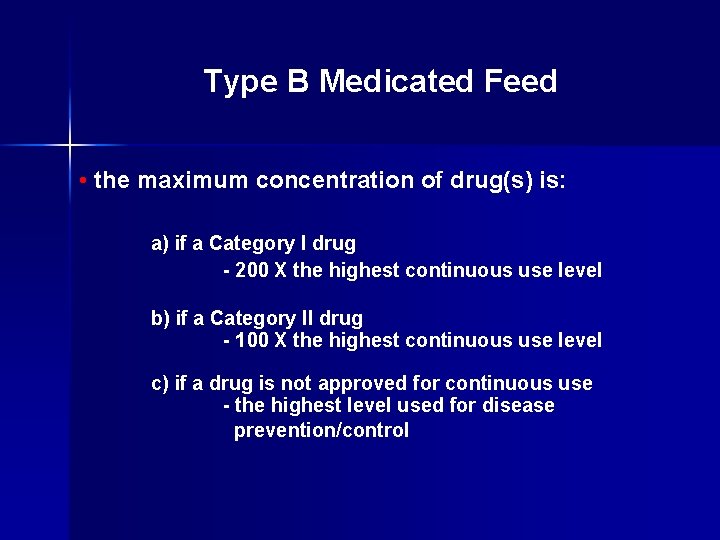
Type B Medicated Feed • the maximum concentration of drug(s) is: a) if a Category I drug - 200 X the highest continuous use level b) if a Category II drug - 100 X the highest continuous use level c) if a drug is not approved for continuous use - the highest level used for disease prevention/control

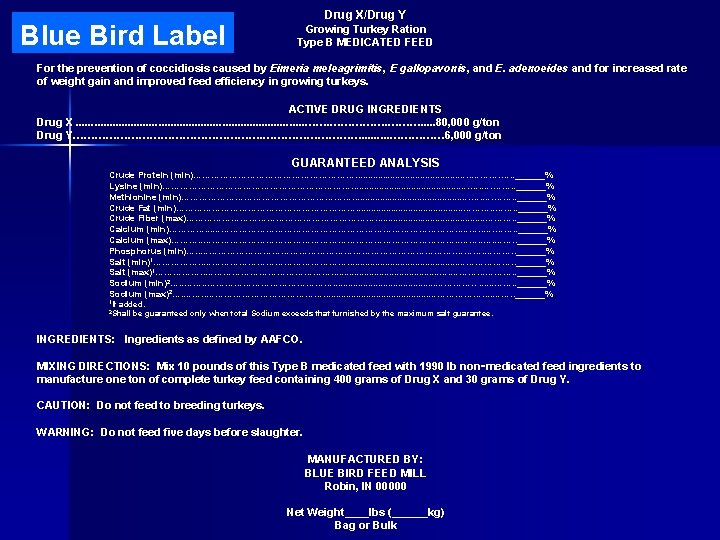
Components of a Type B Blue Bird Label • product name • purpose or indications for use • active ingredients • guaranteed analysis • ingredients • mixing directions • warning and caution sections (if any) • name and address of manufacturer • net weight statement

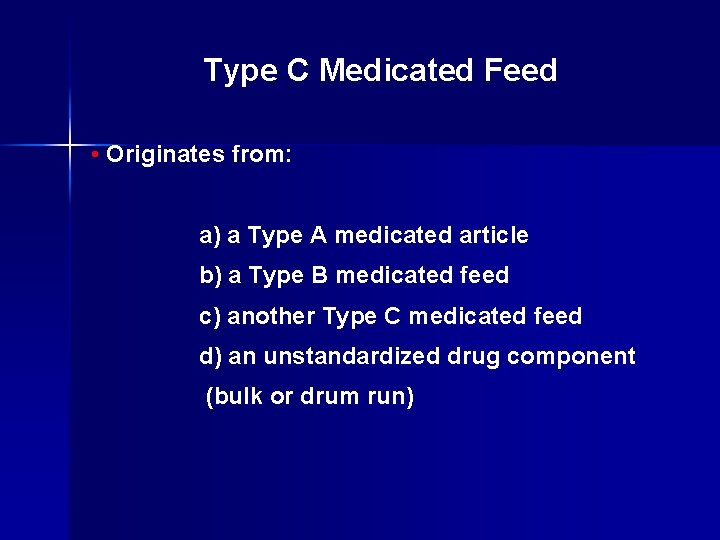
Type C Medicated Feed • Originates from: a) a Type A medicated article b) a Type B medicated feed c) another Type C medicated feed d) an unstandardized drug component (bulk or drum run)

Type C Medicated Feed • is intended for feeding as: a) the complete feed b) ‘top dressed’ c) ‘free choice’

Components of a Type C Blue Bird Label • product name • purpose or indications for use • active ingredients • guaranteed analysis • ingredients • feeding directions • warning and caution sections (if any) • name and address of manufacturer • net weight statement

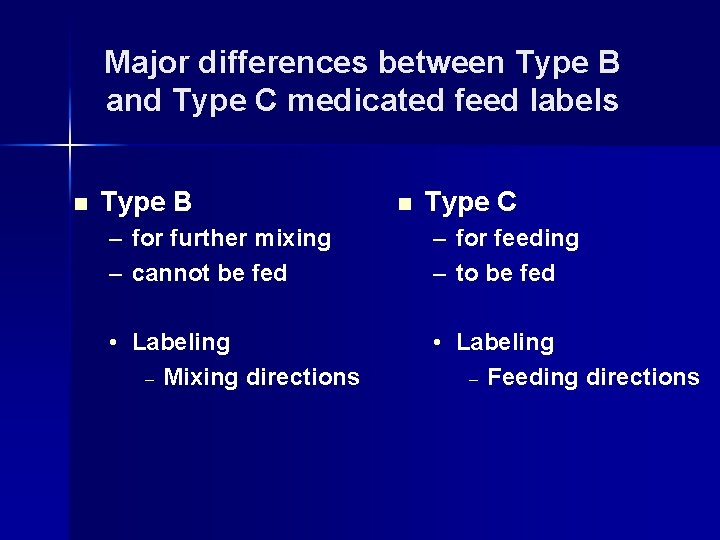
Major differences between Type B and Type C medicated feed labels n Type B n Type C – for further mixing – cannot be fed – for feeding – to be fed • Labeling – Mixing directions • Labeling – Feeding directions

FDA’s animal drug approval process (basics)

New Animal Drug Application (NADA) - Required for: - the manufacture of all Type A medicated articles

The Federal Food, Drug, and Cosmetic Act • mandates that a new animal drug may not be sold in interstate commerce unless it is the subject of a New Animal Drug Application (NADA)

The Federal Food, Drug, and Cosmetic Act • What does an approved NADA mean?

The Federal Food, Drug, and Cosmetic Act • What does an approved NADA mean? • The product is safe and effective for its intended use

The Federal Food, Drug, and Cosmetic Act • What does an approved NADA mean? • The product is safe and effective for its intended use • The methods, facilities and controls used for the manufacturing, processing and packaging of the drug are adequate to preserve its identity, strength, quality and purity

The Federal Food, Drug, and Cosmetic Act • Effectiveness Based on substantial evidence consisting of one or more adequate and well controlled investigations, such as - a study in a target species - a study in laboratory animals - a bioequivalence study - an in vitro study

The Federal Food, Drug, and Cosmetic Act • Safety - Adequate tests by all methods reasonably applicable show that the drug is safe for use under the conditions prescribed, recommended, or suggested in the proposed labeling

The Federal Food, Drug, and Cosmetic Act • NADA - is a systematic approach to document evidence that drug products are safe and effective - consists of the drug, the packaging, and the labeling

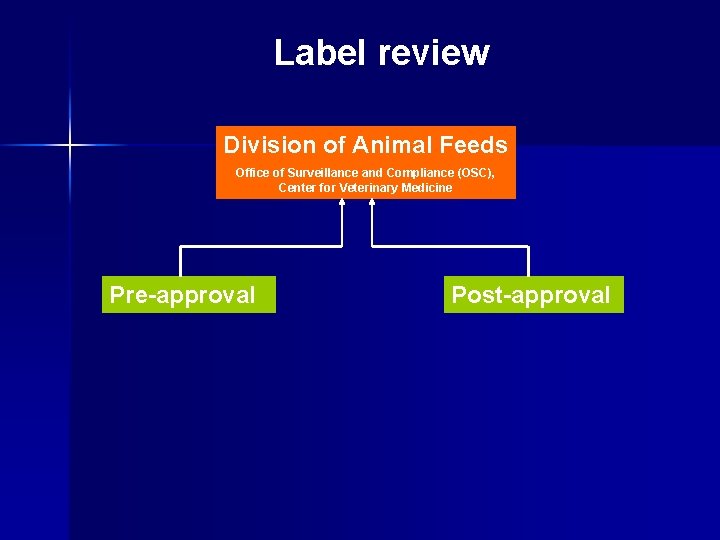
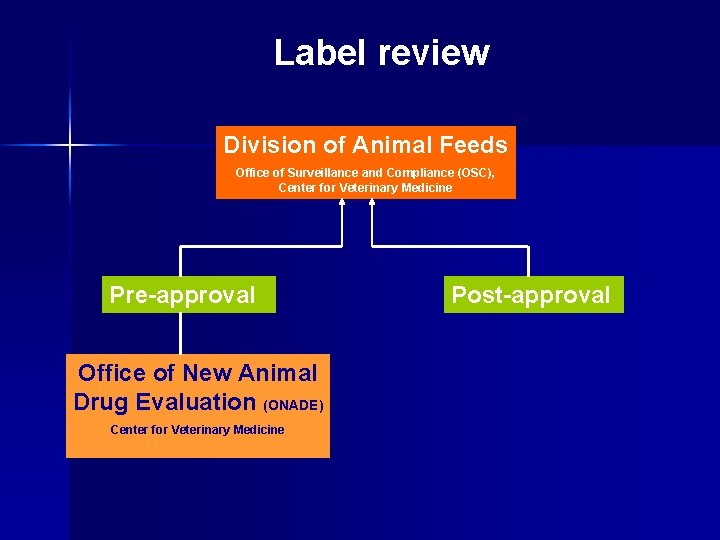
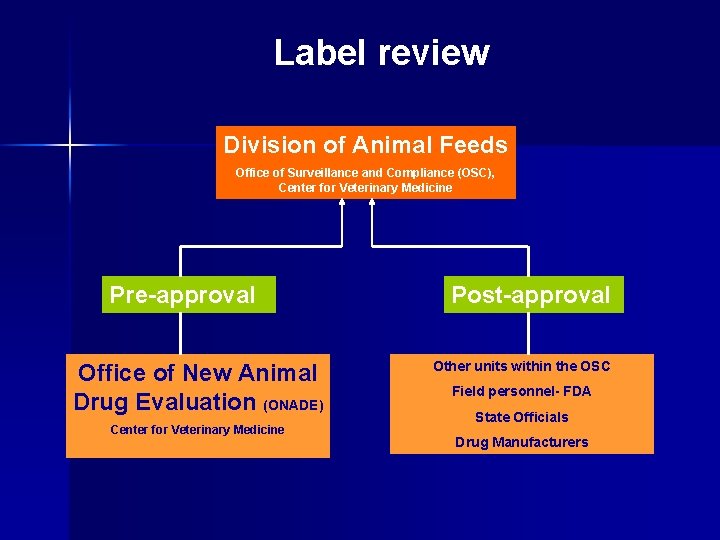
Label review Division of Animal Feeds Office of Surveillance and Compliance (OSC), Center for Veterinary Medicine

Label review Division of Animal Feeds Office of Surveillance and Compliance (OSC), Center for Veterinary Medicine Pre-approval Post-approval

Label review Division of Animal Feeds Office of Surveillance and Compliance (OSC), Center for Veterinary Medicine Pre-approval Office of New Animal Drug Evaluation (ONADE) Center for Veterinary Medicine Post-approval

Label review Division of Animal Feeds Office of Surveillance and Compliance (OSC), Center for Veterinary Medicine Pre-approval Office of New Animal Drug Evaluation (ONADE) Center for Veterinary Medicine Post-approval Other units within the OSC Field personnel- FDA State Officials Drug Manufacturers

Labeling of Medicated Products

NADA Regulation n 21 CFR 514. 1(b)(3)(v) – Labeling for new animal drugs intended for use in the manufacture of medicated feeds shall include: n n ( a ) Specimens of labeling to be used for such new animal drug with adequate directions for the manufacture and use of finished feeds for all conditions for which the new animal drug is intended, recommended, or suggested in any of the labeling, including advertising, sponsored by the applicant. Ingredient labeling may utilize collective names as provided in § 501. 110 of this chapter. ( b ) Representative labeling proposed to be used for Type B and Type C medicated feeds containing the new animal drug.

Medicated Products • Type A medicated article • Type B medicated feed • Type C medicated feed

Label Review n Types of labels Medicated Product Pre-approval Post-approval Type A medicated article Brand Type B medicated feed Blue Bird Brand Type C medicated feed Blue Bird Brand

Blue Bird Label Drug X/Drug Y Growing Turkey Ration Type B MEDICATED FEED For the prevention of coccidiosis caused by Eimeria meleagrimitis, E gallopavonis, and E. adenoeides and for increased rate of weight gain and improved feed efficiency in growing turkeys. ACTIVE DRUG INGREDIENTS Drug X. . . . . ……………. . . 80, 000 g/ton Drug Y………………………. . …………… 6, 000 g/ton GUARANTEED ANALYSIS Crude Protein (min)………………………. . . . . ………. …. . ______% Lysine (min)……………………………. . . …. ……. . ______% Methionine (min)…………………………. . . …. ……. . ______% Crude Fat (min)…………………………. . . ………. . ______% Crude Fiber (max)……………………………. . . ………. . ______% Calcium (min)…………. . . ……………………………………. . …. ……. . ______% Calcium (max)………. . ……………………………………. . …. ……. . ______% Phosphorus (min)…. . ……………………………………. . ______% Salt (min)1……………. . ……………………. . . ………………. . ______% Salt (max)1……………. . …………………. . . …. ………. . ______% Sodium (min)2…. . ……………………. . . . . …………. . . . ……. . ______% Sodium (max)2…. …………………. . . . . ………………. . ______% 1 If added. be guaranteed only when total Sodium exceeds that furnished by the maximum salt guarantee. 2 Shall INGREDIENTS: Ingredients as defined by AAFCO. MIXING DIRECTIONS: Mix 10 pounds of this Type B medicated feed with 1990 lb non‑medicated feed ingredients to manufacture one ton of complete turkey feed containing 400 grams of Drug X and 30 grams of Drug Y. CAUTION: Do not feed to breeding turkeys. WARNING: Do not feed five days before slaughter. MANUFACTURED BY: BLUE BIRD FEED MILL Robin, IN 00000 Net Weight____lbs (______kg) Bag or Bulk

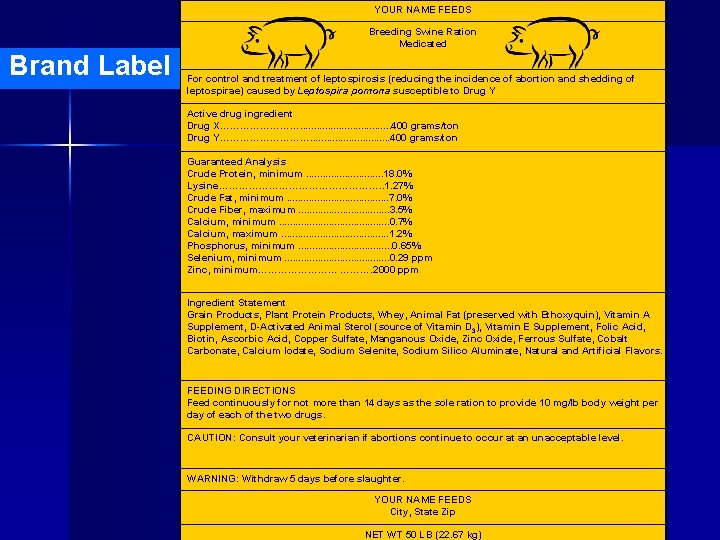
YOUR NAME FEEDS Breeding Swine Ration Medicated Brand Label For control and treatment of leptospirosis (reducing the incidence of abortion and shedding of leptospirae) caused by Leptospira pomona susceptible to Drug Y Active drug ingredient Drug X…………. . . . 400 grams/ton Drug Y……………. . . . 400 grams/ton Guaranteed Analysis Crude Protein, minimum. . . . 18. 0% Lysine……………………. . 1. 27% Crude Fat, minimum. . . . . 7. 0% Crude Fiber, maximum. . . . 3. 5% Calcium, minimum. . . . . 0. 7% Calcium, maximum. . . . . 1. 2% Phosphorus, minimum. . . . 0. 65% Selenium, minimum. . . . . 0. 29 ppm Zinc, minimum………… ………. 2000 ppm Ingredient Statement Grain Products, Plant Protein Products, Whey, Animal Fat (preserved with Ethoxyquin), Vitamin A Supplement, D-Activated Animal Sterol (source of Vitamin D 3), Vitamin E Supplement, Folic Acid, Biotin, Ascorbic Acid, Copper Sulfate, Manganous Oxide, Zinc Oxide, Ferrous Sulfate, Cobalt Carbonate, Calcium Iodate, Sodium Selenite, Sodium Silico Aluminate, Natural and Artificial Flavors. FEEDING DIRECTIONS Feed continuously for not more than 14 days as the sole ration to provide 10 mg/lb body weight per day of each of the two drugs. CAUTION: Consult your veterinarian if abortions continue to occur at an unacceptable level. WARNING: Withdraw 5 days before slaughter. YOUR NAME FEEDS City, State Zip NET WT 50 LB (22. 67 kg)

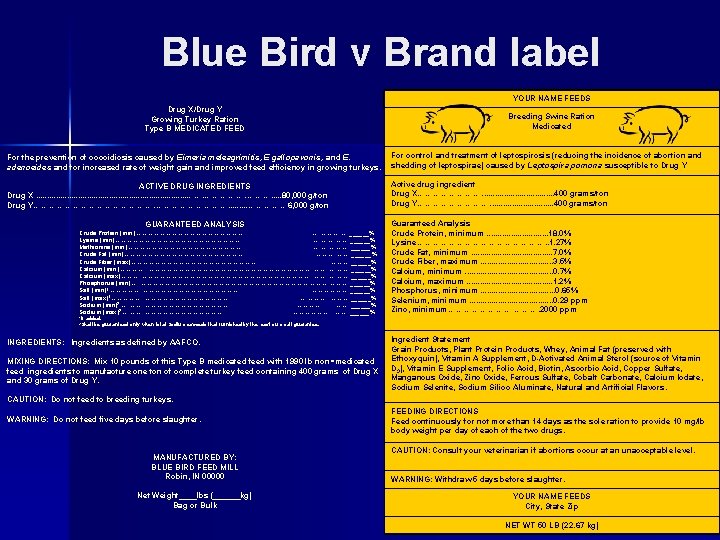
Blue Bird v Brand label YOUR NAME FEEDS Drug X/Drug Y Growing Turkey Ration Type B MEDICATED FEED Breeding Swine Ration Medicated For the prevention of coccidiosis caused by Eimeria meleagrimitis, E gallopavonis, and E. adenoeides and for increased rate of weight gain and improved feed efficiency in growing turkeys. For control and treatment of leptospirosis (reducing the incidence of abortion and shedding of leptospirae) caused by Leptospira pomona susceptible to Drug Y ACTIVE DRUG INGREDIENTS Drug X. . . . . ……. ………. . . 80, 000 g/ton Drug Y……………………. . …………… 6, 000 g/ton Active drug ingredient Drug X…………. . . . 400 grams/ton Drug Y……………. . . . 400 grams/ton GUARANTEED ANALYSIS Crude Protein (min)………………………. . . . . ………. …. . ______% Lysine (min)……………………………. . . …. ……. . ______% Methionine (min)…………………………. . . …. ……. . ______% Crude Fat (min)…………………………. . . ………. . ______% Crude Fiber (max)……………………………. . . ………. . ______% Calcium (min)…………. . . ……………………………………. . …. ……. . ______% Calcium (max)………. . ……………………………………. . …. ……. . ______% Phosphorus (min)…. . ……………………………………. . ______% Salt (min)1……………. . ……………………. . . ………………. . ______% Salt (max)1……………. . …………………. . . …. ………. . ______% Sodium (min)2…. . ……………………. . . . . …………. . . . ……. . ______% Sodium (max)2…. …………………. . . . . ………………. . ______% 1 If Guaranteed Analysis Crude Protein, minimum. . . . 18. 0% Lysine……………………. . 1. 27% Crude Fat, minimum. . . . . 7. 0% Crude Fiber, maximum. . . . 3. 5% Calcium, minimum. . . . . 0. 7% Calcium, maximum. . . . . 1. 2% Phosphorus, minimum. . . . 0. 65% Selenium, minimum. . . . . 0. 29 ppm Zinc, minimum………… ………. 2000 ppm added. be guaranteed only when total Sodium exceeds that furnished by the maximum salt guarantee. 2 Shall INGREDIENTS: Ingredients as defined by AAFCO. MIXING DIRECTIONS: Mix 10 pounds of this Type B medicated feed with 1990 lb non ‑medicated feed ingredients to manufacture one ton of complete turkey feed containing 400 grams of Drug X and 30 grams of Drug Y. Ingredient Statement Grain Products, Plant Protein Products, Whey, Animal Fat (preserved with Ethoxyquin), Vitamin A Supplement, D-Activated Animal Sterol (source of Vitamin D 3), Vitamin E Supplement, Folic Acid, Biotin, Ascorbic Acid, Copper Sulfate, Manganous Oxide, Zinc Oxide, Ferrous Sulfate, Cobalt Carbonate, Calcium Iodate, Sodium Selenite, Sodium Silico Aluminate, Natural and Artificial Flavors. CAUTION: Do not feed to breeding turkeys. WARNING: Do not feed five days before slaughter. MANUFACTURED BY: BLUE BIRD FEED MILL Robin, IN 00000 Net Weight____lbs (______kg) Bag or Bulk FEEDING DIRECTIONS Feed continuously for not more than 14 days as the sole ration to provide 10 mg/lb body weight per day of each of the two drugs. CAUTION: Consult your veterinarian if abortions occur at an unacceptable level. WARNING: Withdraw 5 days before slaughter. YOUR NAME FEEDS City, State Zip NET WT 50 LB (22. 67 kg)

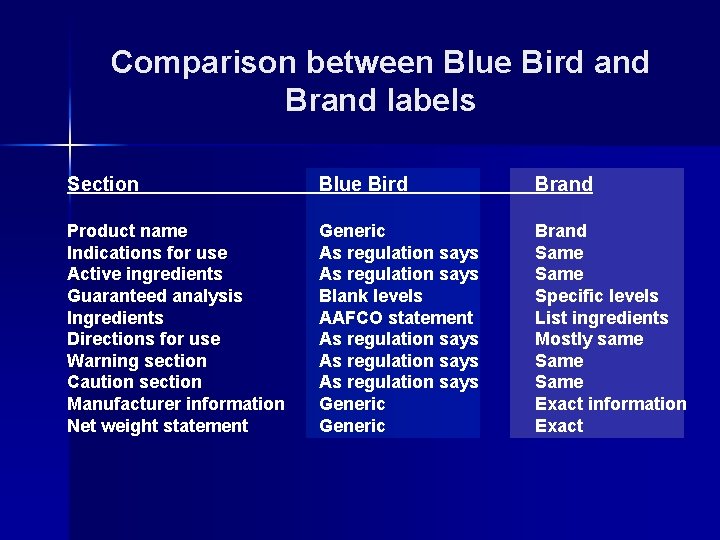
Comparison between Blue Bird and Brand labels Section Blue Bird Brand Product name Indications for use Active ingredients Guaranteed analysis Ingredients Directions for use Warning section Caution section Manufacturer information Net weight statement Generic As regulation says Blank levels AAFCO statement As regulation says Generic Brand Same Specific levels List ingredients Mostly same Same Exact information Exact

 Srednji kurs za period
Srednji kurs za period Dragan milovanovic
Dragan milovanovic Učitelju vrati mi klikere
Učitelju vrati mi klikere Dragan blanusa
Dragan blanusa Feodor dragan
Feodor dragan Dragan masulovic
Dragan masulovic Jkp bvk
Jkp bvk Dragan rangelov
Dragan rangelov Dragan uletilovic sudija
Dragan uletilovic sudija Dragan prole
Dragan prole Public health monash
Public health monash Classification of root canal sealers
Classification of root canal sealers Monophasic dosage form
Monophasic dosage form Simple evacuant enema
Simple evacuant enema Types of powders in pharmacy
Types of powders in pharmacy Examples of granules
Examples of granules Permanent medicated filling
Permanent medicated filling Shampooing encompasses three different processes
Shampooing encompasses three different processes Method of preparation of elixirs
Method of preparation of elixirs Programming rss feeds
Programming rss feeds Can i have one of your chips figurative language
Can i have one of your chips figurative language Don't bite the hand that feeds you figurative language
Don't bite the hand that feeds you figurative language Thomas feeds his dog
Thomas feeds his dog Tmg feeds
Tmg feeds Classification of animal feed
Classification of animal feed Don't bite the hand that feeds you figurative language
Don't bite the hand that feeds you figurative language Mimaropa region
Mimaropa region A flag wags like a fishhook there in the sky
A flag wags like a fishhook there in the sky Avamar overview
Avamar overview Overview of the dentitions chapter 11
Overview of the dentitions chapter 11 I company
I company Introduce the
Introduce the Consulting industry overview
Consulting industry overview Solar massachusetts renewable target (smart) program
Solar massachusetts renewable target (smart) program Overview of the major systemic arteries
Overview of the major systemic arteries Overview text
Overview text Iptv technoligies
Iptv technoligies Methodologies for cross-domain data fusion: an overview
Methodologies for cross-domain data fusion: an overview 7 series fpga
7 series fpga Intro to hrm
Intro to hrm Lesson overview
Lesson overview Energy management outsourcing
Energy management outsourcing Software testing overview
Software testing overview Cmmi overview
Cmmi overview Overview of http
Overview of http Fsma overview
Fsma overview Computer memory system overview
Computer memory system overview Overview of cellular respiration
Overview of cellular respiration Management overview
Management overview Overview of government accounting
Overview of government accounting Dna purification overview
Dna purification overview