Medical Physics at A Level XRays Contents Uses



























- Slides: 49

Medical Physics at A Level X-Rays

Contents • • • Uses Production of X-Rays Brehmsstrahlung radiation Characteristic radiation The X-ray tube Rotating anode Intensifying screen Barium Meal Barium Enema

X-Rays are: • high frequency, high energy electromagnetic rays. • undetectable by the human senses • very penetrating • low localised ionization. • generated when high energy electrons struck a metal target

Uses: • Imaging for detection of broken bones or tumors: – simple X-ray, – CT scanning, – barium meal scanning. • High energy rays for treatment of cancer (destroying cells) - radiotherapy • As incident beams in 'material characterization technologies' (ways of finding out the properties of materials), such as X-ray Diffraction, X-ray Photoelectron Spectroscopy and Auger Electron Spectroscopy.

From the syllabus: • Physical principles of the production of X-rays: – rotating-anode X-ray tube; – methods of controlling the beam intensity, – the photon energy, – the image sharpness and contrast and – the patient dose

Production of X-rays • X-rays are generated when high energy electrons struck a metal target. • The kinetic energy of the electron is transformed into electromagnetic energy. • Two kinds of x-rays are generated during this process. – Brehmsstrahlung radiation, also called "braking radiation" or white x-rays is produced due to electron deceleration. – Characteristic x-rays are also produced when electrons in target metal make transitions between atomic energy levels.

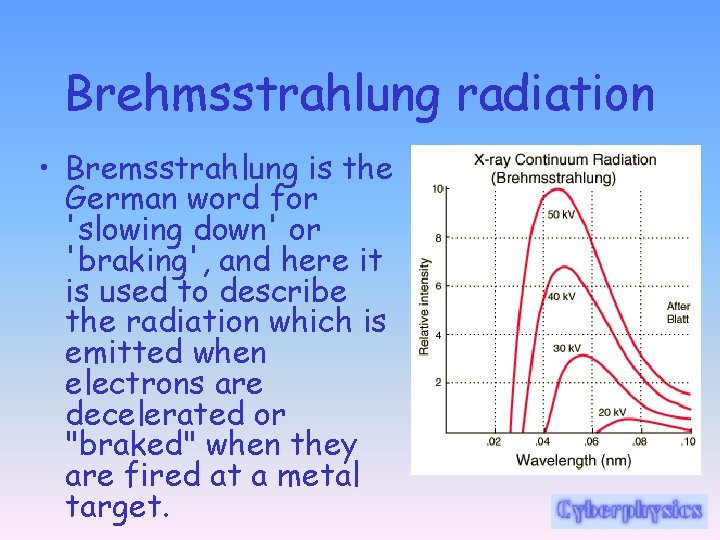
Brehmsstrahlung radiation • Bremsstrahlung is the German word for 'slowing down' or 'braking', and here it is used to describe the radiation which is emitted when electrons are decelerated or "braked" when they are fired at a metal target.

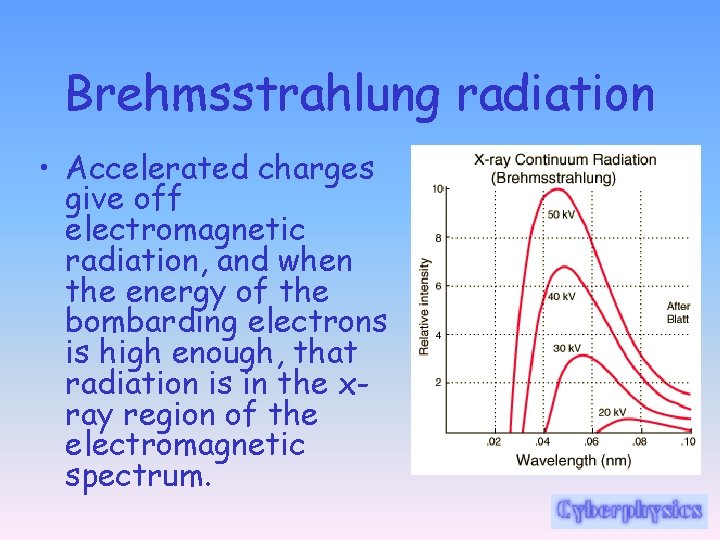
Brehmsstrahlung radiation • Accelerated charges give off electromagnetic radiation, and when the energy of the bombarding electrons is high enough, that radiation is in the xray region of the electromagnetic spectrum.

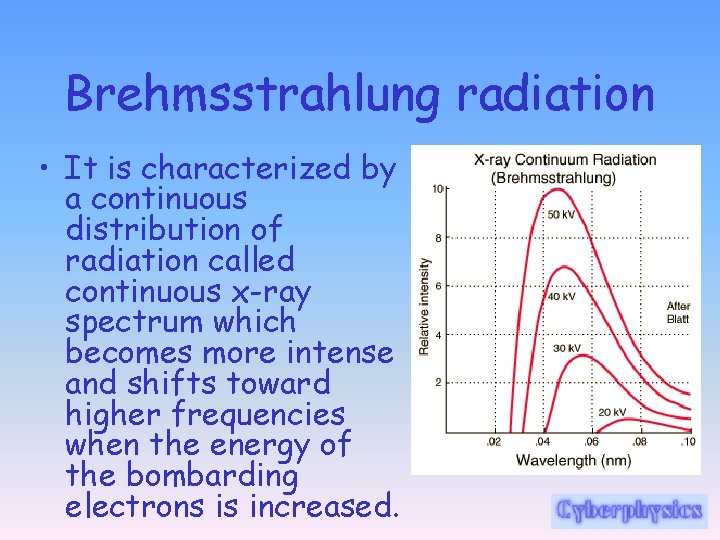
Brehmsstrahlung radiation • It is characterized by a continuous distribution of radiation called continuous x-ray spectrum which becomes more intense and shifts toward higher frequencies when the energy of the bombarding electrons is increased.

Brehmsstrahlung radiation • A projectile electron can lose any amount of its kinetic energy in an interaction with the nucleus of a target atom. • So the bremsstrahlung radiation associated with the loss can take on a corresponding range of values. • For example, an electron with kinetic energy of 70 ke. V can lose all, none, or any intermediate level of that kinetic energy in a bremsstrahlung interaction.

Brehmsstrahlung radiation • The Bremsstrahlung X-ray produced can have an energy in the range of 0 to 70 ke. V. • Here, 70 ke. V is the energy that corresponds to the cut off wavelength (smallest wavelength - highest frequency therefore the highest possible energy - use E=hf=hc/l to calculate it). This is different from the production of characteristic x-rays that have specific energies.

Characteristic X-rays • Characteristic X-rays are produced by transitions of orbital electrons from outer to inner shells. • Bombarding electrons can release electrons from inner energy level orbits. • Higher electrons can then fall into the vacancy and if the energy gap between the levels is great enough X-rays will be produced.

Characteristic X-rays • Since the electron binding energy for every element is different, the characteristic X-rays produced in the various elements are also different. • This type of X-radiation is called characteristic radiation because it has precisely fixed, or discrete, energies and that these energies are characteristic of the differences between electron binding energies of a particular element. • The effective energy characteristic Xrays increases with increasing atomic number of the target element.

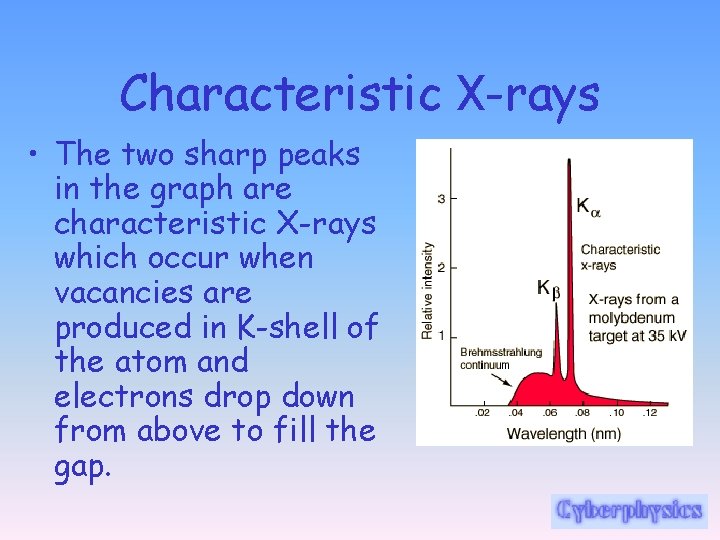
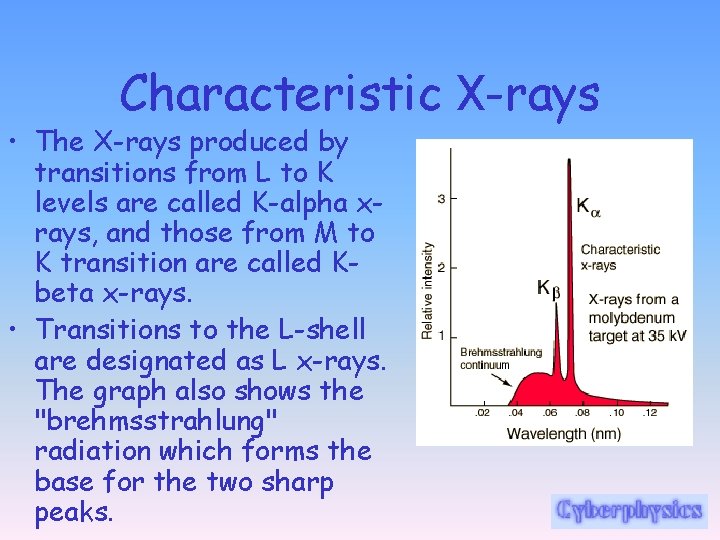
Characteristic X-rays • The two sharp peaks in the graph are characteristic X-rays which occur when vacancies are produced in K-shell of the atom and electrons drop down from above to fill the gap.

Characteristic X-rays • The X-rays produced by transitions from L to K levels are called K-alpha xrays, and those from M to K transition are called Kbeta x-rays. • Transitions to the L-shell are designated as L x-rays. The graph also shows the "brehmsstrahlung" radiation which forms the base for the two sharp peaks.

Example to calculate the emitted x-ray energy: For tungsten, K electrons have binding energies of 69. 5 ke. V, and L electrons are bound by 12. 1 ke. V. A K-shell electron is removed from a tungsten atom and is replaced by an L shell electron. What is the energy of the characteristic X-ray that is emitted (in ke. V)? ANSWER: 57. 4 ke. V (the difference in the energies of the shells).

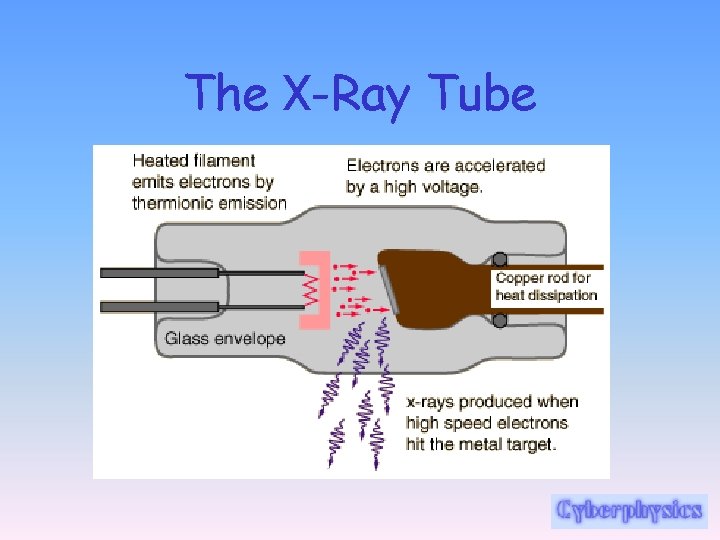
The X-Ray Tube

The X-Ray Tube: Thermionic Emission • Electrons are produced by thermionic emission in the cathode. This is heated by a relatively low voltage supply. At a cathode current of 100 m. A, for example, 6 x 1017 electrons travel from the cathode to the anode of the X-ray tube every second.

The X-Ray Tube: electron missiles! • They are accelerated from the cathode to anode across a high voltage. As the kinetic energy of the electrons increases, both the intensity (number of x-rays) and the energy (their ability to penetrate) of the X-rays produced are increased.

Rotating anode • To increase the output, tubes with a rotating anode of a diameter up to 200 mm for a better dissipation of heat are used. • The anode is accelerated up to 9000 rpm within less than 1 second. • Heat buildup will rapidly damage the target unless some provision is made for its dissipation.

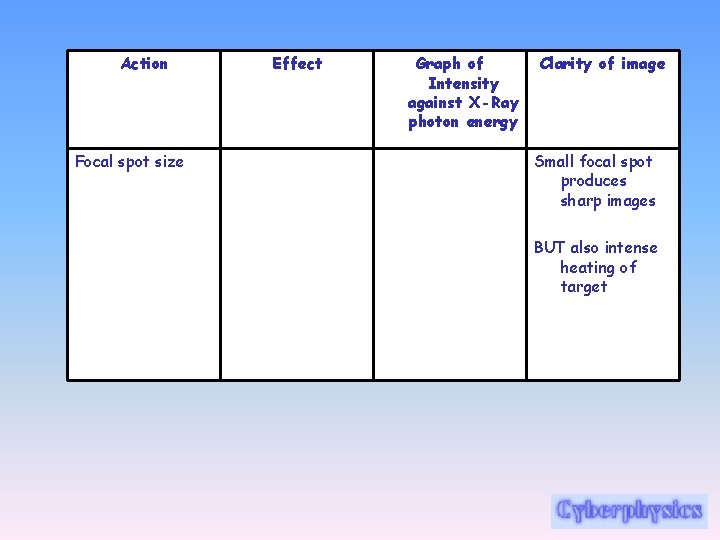
Rotating anode • The most common solution to the problem in medical x-ray tubes is to mount the anode target on the armature of an electric motor so that the target becomes a spinning disc which has the capacity to absorb the heat over a large area even though the actual focal spot is quite small. • The size of the focal spot affects the sharpness of the radiograph. As the focal spot is enlarged, the radiograph becomes less sharp… therefore a spinning anode is better than having a bigger focal spot

The X-Ray Tube: X-Ray Production • When these electrons bombard on the heavy metal atoms of the target, they interact with these atoms and transfer their kinetic energy to the target. • These interactions occur within a very small depth of penetration into the target. • As they occur, the electrons slow down (brake!) and finally come nearly to rest, at which time they can be conducted through the x-ray anode assembly and out into the associated electronic circuitry

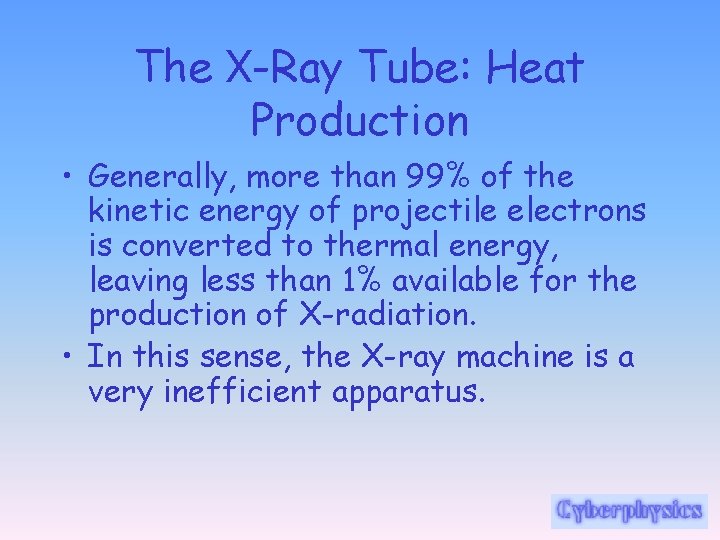
The X-Ray Tube: Energy Changes • The interactions result in the conversion of kinetic energy into thermal energy and electromagnetic energy in the form of X-rays. • By far, most of the kinetic energy is converted into heat. • The electrons interact with the outer -shell electrons of the target atoms but do not transfer sufficient energy to these outer-shell electrons to ionize them.

The X-Ray Tube: Energy Changes • Rather, the outer-shell electrons are simply raised to an excited, or higher, energy level. The outer-shell electrons immediately drop back to their normal energy state with the emission of infrared radiation. • The constant excitation and restabilization of outer-shell electrons is responsible for the heat generated in the anodes of X-ray tubes.

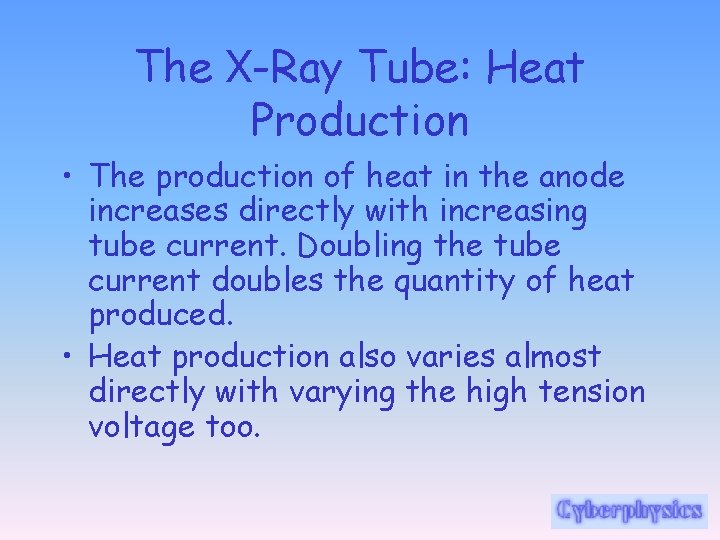
The X-Ray Tube: Heat Production • Generally, more than 99% of the kinetic energy of projectile electrons is converted to thermal energy, leaving less than 1% available for the production of X-radiation. • In this sense, the X-ray machine is a very inefficient apparatus.

The X-Ray Tube: Heat Production • The production of heat in the anode increases directly with increasing tube current. Doubling the tube current doubles the quantity of heat produced. • Heat production also varies almost directly with varying the high tension voltage too.

The X-Ray Tube: efficiency • The efficiency of X-ray production is independent of the tube current. • Regardless of what m. A is selected, the efficiency of X-ray production remains constant. • The efficiency of X-ray production increases with increasing projectileelectron energy. • At 60 k. Vp, only 0. 5% of the electron kinetic energy is converted to X-rays; at 120 Me. V, it is 70%.

The X-Ray Tube: Target Material • needs to have a high Z (proton number) so that transitions of high enough energy to emit X-ray radiation are possible • needs to have a high melting point because so much heat energy is produced. • tungsten is ideal (Molybdenum for softer X -rays needed for breast X-rays)

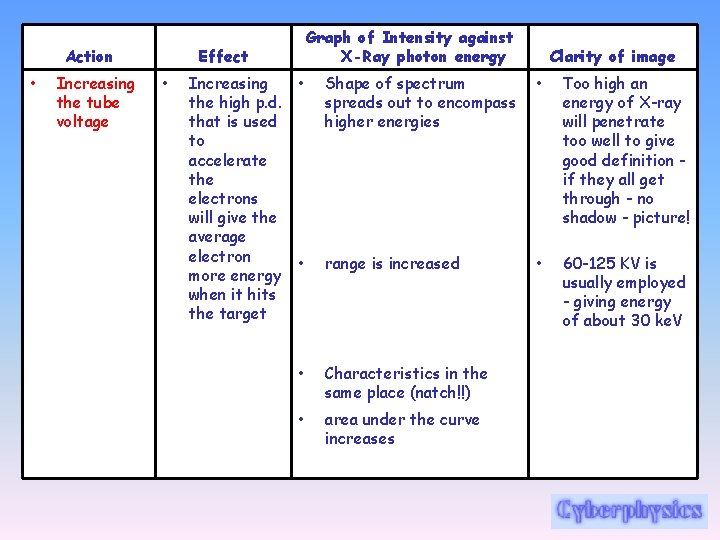
Action • Increasing the tube voltage Graph of Intensity against X-Ray photon energy Effect • Increasing the high p. d. that is used to accelerate the electrons will give the average electron more energy when it hits the target Clarity of image • Shape of spectrum spreads out to encompass higher energies • Too high an energy of X-ray will penetrate too well to give good definition if they all get through - no shadow - picture! • range is increased • 60 -125 KV is usually employed - giving energy of about 30 ke. V • Characteristics in the same place (natch!!) • area under the curve increases

Action AC/DC voltage (AC necessary to get higher voltages - can use transformers! DC acquired by electronic rectiication and 'smoothing' circuitry) Effect Electrons produced by thermionic emission only accelerated across half of the time! Graph of Intensity against X-Ray photon energy graphs for both are the same except the DC one is double the intensity throughout (only accelerated across to target on half of the wave).

Action Effect Graph of Intensity against X-Ray photon energy Clarity of image Increasing the tube current (low voltage one!) Increases the rate of thermionic emission - more electrons hit the target - more Xrays produced. Shape of spectrum remains the same Overall increase of exposure of film range is the same but bigger dose to patient! Characteristics in the same place (natch!!) more heating of the target area under the curve increases

Action Increasing exposure time Effect Graph of Intensity against X-Ray photon energy Clarity of image Overall increase of exposure of film but bigger dose to patient! more heating of the target risk of blur due to movement of patient - big problem with organs that cannot be constrained.

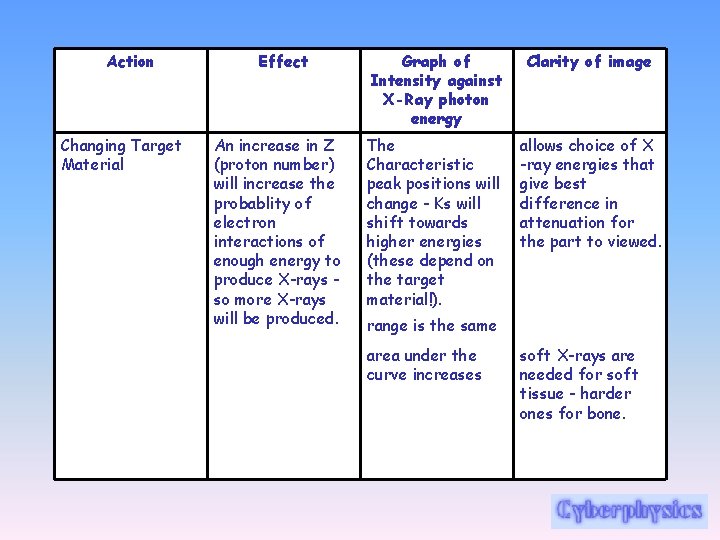
Action Changing Target Material Effect An increase in Z (proton number) will increase the probablity of electron interactions of enough energy to produce X-rays so more X-rays will be produced. Graph of Intensity against X-Ray photon energy Clarity of image The Characteristic peak positions will change - Ks will shift towards higher energies (these depend on the target material!). allows choice of X -ray energies that give best difference in attenuation for the part to viewed. range is the same area under the curve increases soft X-rays are needed for soft tissue - harder ones for bone.

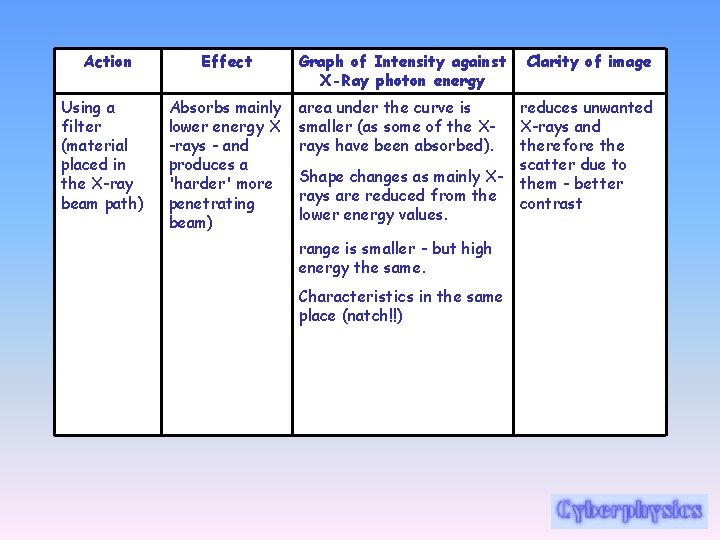
Action Using a filter (material placed in the X-ray beam path) Effect Absorbs mainly lower energy X -rays - and produces a 'harder' more penetrating beam) Graph of Intensity against X-Ray photon energy area under the curve is smaller (as some of the Xrays have been absorbed). Shape changes as mainly Xrays are reduced from the lower energy values. range is smaller - but high energy the same. Characteristics in the same place (natch!!) Clarity of image reduces unwanted X-rays and therefore the scatter due to them - better contrast

Action Reducing beam size Effect Graph of Intensity against X-Ray photon energy Clarity of image less scatter better contrast especially if a collimator is used (lead grid that only allows Xrays in a particular direction to get through.

Action Artificial Contrast Media Effect See barium meal/enema Graph of Intensity against X-Ray photon energy Clarity of image Clearly outlines the inner surface of internal bodily organs by coating them in a radioopaque material barium sulphate.

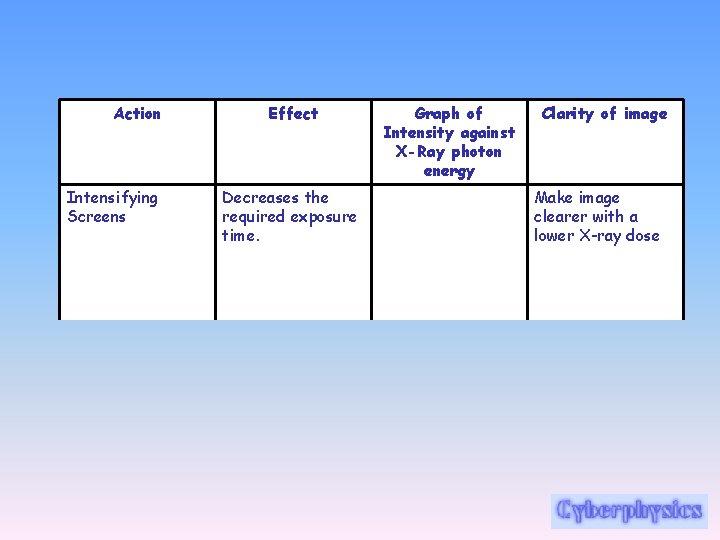
Action Intensifying Screens Effect Decreases the required exposure time. Graph of Intensity against X-Ray photon energy Clarity of image Make image clearer with a lower X-ray dose

Action Focal spot size Effect Graph of Intensity against X-Ray photon energy Clarity of image Small focal spot produces sharp images BUT also intense heating of target

Intensifying Screens • X-ray and other photographic films are sensitive to the direct action of the x-rays, but the photographic effect can be increased very appreciably, and exposure time can be decreased by the use of an intensifying screen in contact with each side of the film. • One form of intensifying screen consists of lead foil, or a thin layer of a lead compound evenly coated on a paper backing. Under the excitation of x-rays of short wavelength and gamma rays, lead is a good emitter of electrons, which expose the sensitive film, thus increasing the total photographic effect.

Intensifying Screens • Another form of intensifying screen consists of a powdered fluorescent chemical--for example, calcium tungstate, mixed with a suitable binder and coated on cardboard or plastic. Its action depends on the fact that it converts some of the x-ray energy into light, to which the film is very sensitive. • The decision as to the type of screen to be used-or whether a screen is to be used at alldepends on a variety of circumstances, and is made by the radiographer.

Types of film • Several special types of x-ray film have been designed for the radiography of materials. Some types work best with lead screens, or without screens. Other types are intended primarily for use with fluorescent intensifying screens.

Types of film • X-ray films are commonly coated with emulsion on both sides of the support (to double the chance of exposure and therefore decrease the dose) --the superposition of the radiographic images of the two emulsion layers doubles the density and hence greatly increases the speed at which the X-Ray image is formed (halving the dose to the patient).

Types of film • X-ray films coated on one side only (single-coated films) are available for use when the superposed images in two emulsions might cause confusion - when a very detailed image of an area is required.

Barium meal • A barium meal is an x-ray examination of the stomach and your oesophagus (gullet). Often pictures of the first part of the small intestine (the duodenum) are also taken. • For the test to be successful the stomach should be as empty as possible and so the patient will probably be asked not to eat or drink anything for six hours before the examination.

Barium meal • The patient will be asked to swallow some fizzy tablets or granules, with a little water. These will expand the stomach with gas which makes it easier to get a clear view of things. It is very important that the patient does not belch once s/he has taken these. Sometimes s/he if also given an injection of a drug to relax the stomach and stop it moving while the x -rays are taken (this can cause some blurring of vision for an hour or so and if this happens it is best not to drive).

Barium meal • The patient is then given a cup of 'barium' to drink. It is actually barium sulphate (a radiopaque - contrast medium) and the mixture used normally contains defoaming agents and a mixture of constituents that make it have excellent coating characteristics. It is often fruit flavoured and is not at all unpleasant.

Barium meal • The barium shows up on the X-rays as it strongly absorbs X-rays and therefore outlines the gullet and stomach in the X-ray picture. • A number of X-ray pictures will then be taken. This is completely painless. • The examination is usually completed within 30 minutes.

Barium Enema • A barium enema is an x-ray examination which involves filling the large intestine with barium through a tube inserted into the rectum. It is similar to the meal - just inserted into the body the other way round!

Barium Enema • The patient can eat and drink quite normally once the test is completed. The barium will be passed out with your bowel motions during the next few days, it may make motions paler in colour than normal. • The results of the examination will usually be available a few days later.