Medical Nutrition Therapy in Pulmonary Disease Malnutrition and
























































- Slides: 86

Medical Nutrition Therapy in Pulmonary Disease

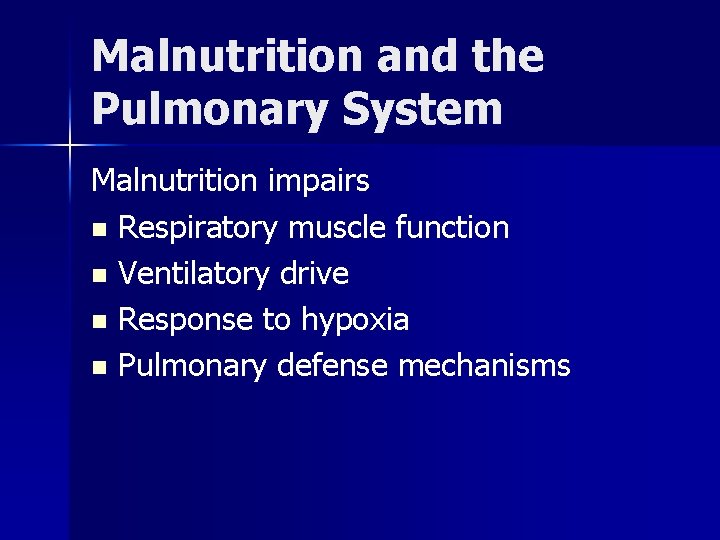
Malnutrition and the Pulmonary System Malnutrition impairs n Respiratory muscle function n Ventilatory drive n Response to hypoxia n Pulmonary defense mechanisms

Effects of Malnutrition in Pts without Lung Disease n n Respiratory muscle strength ↓ by 37% Maximum voluntary ventilation ↓ by 41% (1) Vital capacity (lung volume)↓ 63% (1) Diaphragmatic muscle mass ↓ to 60% of normal in underweight patients who died of other ailments (2) 1. Aurora N, Rochester, D. Am Rev Respir Dis 126: 5 -8, 1982 2. Aurora N, Rochester D. J Appl Physiol: Respirat Environ Exercise physiol 52: 64 -70, 1982

Effects of Malnutrition in Pts with Pulmonary Disease Decreased cough and inability to mobilize secretions n Atelectasis and pneumonia n Prolonged mechanical ventilation and difficulty weaning with prolonged ICU stay n

Effects of Malnutrition in Pts with Pulmonary Disease Altered host immune response and cell -mediated immunity n Contributes to chronic or repeated pulmonary infections n Decreased surfactant production n Decreased lung elasticity n Decreased ability to repair injured lung tissue n


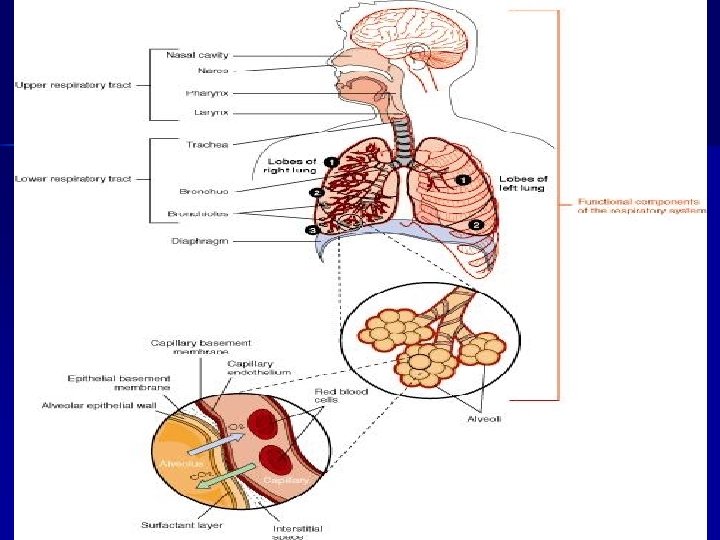
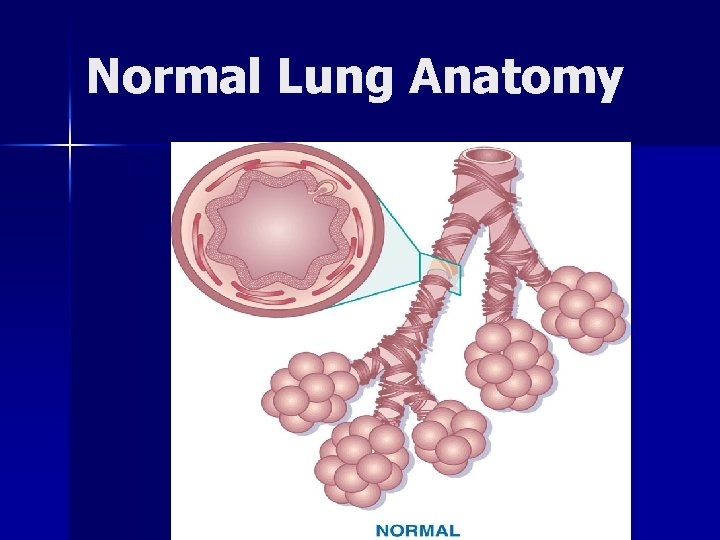
Normal Lung Anatomy

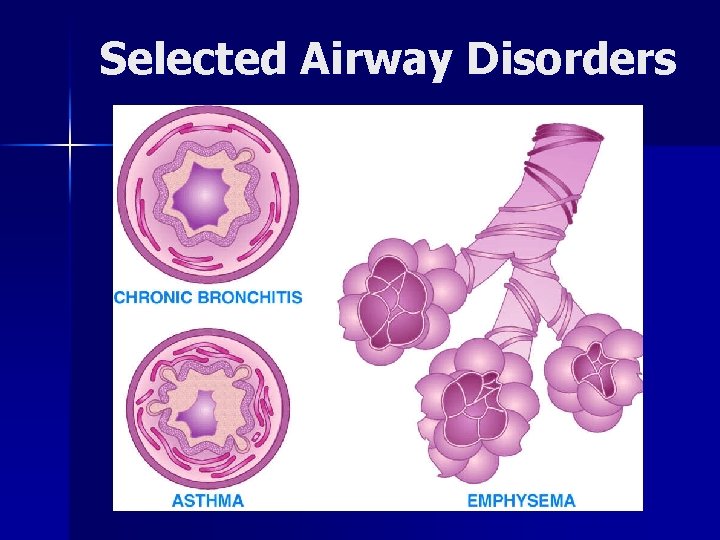
Selected Airway Disorders

Chronic Pulmonary Disorders n Bronchopulmonary n Cystic displasia fibrosis n Tuberculosis n Bronchial asthma n Chronic obstructive pulmonary disease (COPD)

Acute Pulmonary Disorders n Pulmonary aspiration n Pneumonia n Tuberculosis n Cancer of the lung n Acute respiratory distress syndrome n Pulmonary failure

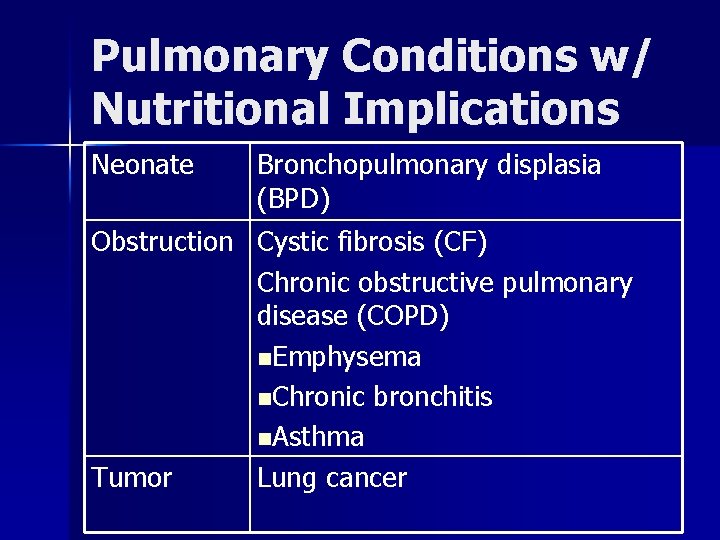
Pulmonary Conditions w/ Nutritional Implications Neonate Bronchopulmonary displasia (BPD) Obstruction Cystic fibrosis (CF) Chronic obstructive pulmonary disease (COPD) n. Emphysema n. Chronic bronchitis n. Asthma Tumor Lung cancer

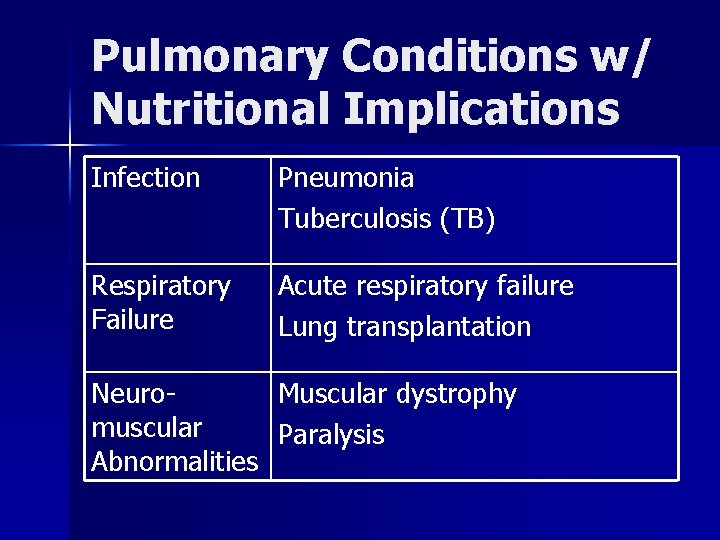
Pulmonary Conditions w/ Nutritional Implications Infection Pneumonia Tuberculosis (TB) Respiratory Failure Acute respiratory failure Lung transplantation Neuromuscular Abnormalities Muscular dystrophy Paralysis

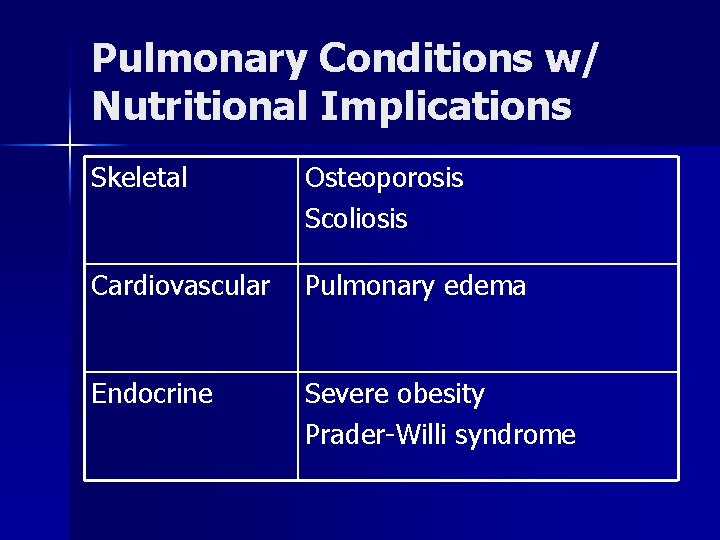
Pulmonary Conditions w/ Nutritional Implications Skeletal Osteoporosis Scoliosis Cardiovascular Pulmonary edema Endocrine Severe obesity Prader-Willi syndrome

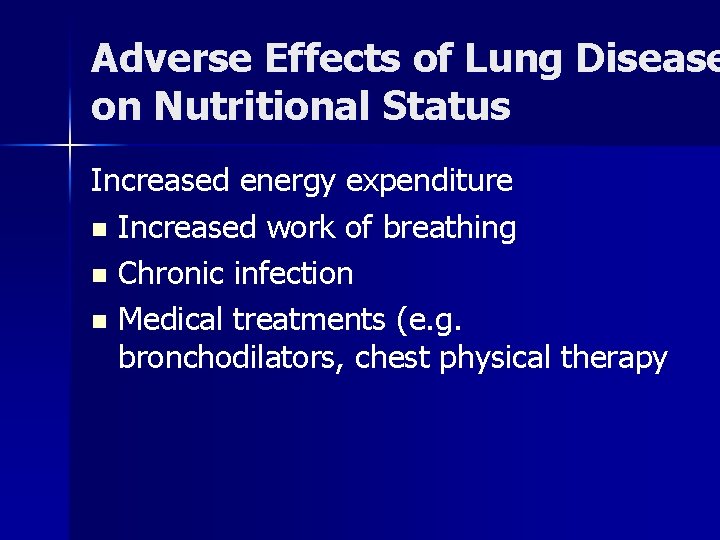
Adverse Effects of Lung Disease on Nutritional Status Increased energy expenditure n Increased work of breathing n Chronic infection n Medical treatments (e. g. bronchodilators, chest physical therapy

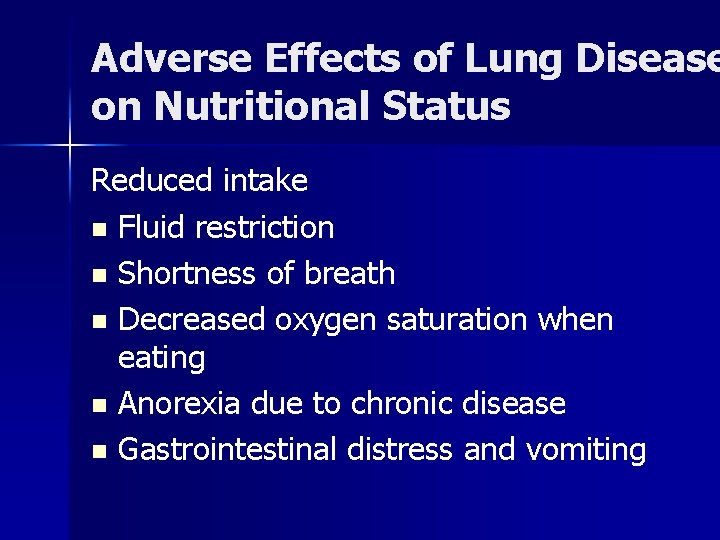
Adverse Effects of Lung Disease on Nutritional Status Reduced intake n Fluid restriction n Shortness of breath n Decreased oxygen saturation when eating n Anorexia due to chronic disease n Gastrointestinal distress and vomiting

Adverse Effects of Lung Disease on Nutritional Status Additional limitations n Difficulty preparing food due to fatigue n Lack of financial resources n Impaired feeding skills (for infants and children) n Altered metabolism

Chronic Lung Disease

Bronchopulmonary Dysplasia: Pathophysiology Chronic lung condition in newborns that often follows respiratory distress syndrome (RDS) and treatment with oxygen n Characterized by broncheolar metaplasia and interstitial fibrosis n Occurs most frequently in infants who are premature or low birth weight n

BPD: Signs and Symptoms Hypercapnea (CO 2 retention) n Tachypnea n Wheezing n Dyspnea n Recurrent respiratory infections n Cor pulmonale (right ventricular enlargement of the heart) n

Growth Failure in BPD Increased energy needs n Inadequate dietary intake n Gastroesophageal reflux n Emotional deprivation n Chronic hypoxia n

Goals of Nutritional Management in BPD Meet nutritional needs n Promote linear growth n Develop age-appropriate feeding skills n Maintain fluid balance n

Energy Needs in BPD n n REE in infants with BPD is 25 -50% higher than in age-matched controls Babies with growth failure may have needs 50% higher Energy needs in acute phase (PN, controlled temperature) 50 -85 kcals/kg Energy needs in convalescence (oral feeds, activity, temperature regulation) as high as 120 -130 kcals/kg

Protein Needs in Babies with BPD Protein: within advised range for infants of comparable postconceptional age n As energy density of the diet is increased by the addition of fat and carbohydrate, protein should still provide 7% or more of total kcals n

Macronutrient Mix in BPD Fat and carbohydrate should be added to formula only after it has been concentrated to 24 kcals/oz to keep protein high enough n Fat provides EFA and energy when tolerance for fluid and carbohydrate is limited n Excess CHO increases RQ and CO 2 output n

Fluid in BPD Infants with BPD may require fluid restriction, sodium restriction, and long term treatment with diuretics n Use of parenteral lipids or calorically dense enteral feeds may help the infant meet energy needs n

Mineral Needs in BPD n n Often driven by the baby’s premature status Lack of mineral stores as a result of prematurity (iron, zinc, calcium) Growth delay Medications: diuretics, bronchodilators, antibiotics, cardiac antiarrhythmics, corticosteroids associated with loss of minerals including chloride, potassium, calcium

Vitamin Needs in BPD Interest in antioxidants, including vitamin A for role in developing epithelial cells of the respiratory tract n Provide intake based on the DRI, including total energy, to promote catchup growth n

Feeding Strategies in BPD Calorically dense formulas or boosted breast milk (monitor fluid status and urinary output) n Small, frequent feedings n Use of a soft nipple n Nasogastric or gastrostomy tube feedings n

Feeding Strategies in Gastroesophageal Reflux Thickened feedings (add rice cereal to formula) n Upright positioning n Medications like antacids or histamine H 2 blockers n Surgical fundoplication n

Long Term Feeding Problems in BPD n n n History of unpleasant oral experiences (intubation, frequent suctioning, recurrent vomiting) History of non-oral feedings Delayed introduction of solids Discomfort or choking associated with eating solids Infants may tire easily while breast-feeding or bottle feeding May require intervention of interdisciplinary feeding team

Cystic Fibrosis Inherited autosomal recessive disorder n 2 -5% of the white population are heterozygous n CF incidence of 1: 2500 live births n 30, 000 people treated at CF centers in the U. S. n Survival is improving; median age of patients has exceeded 30 years n

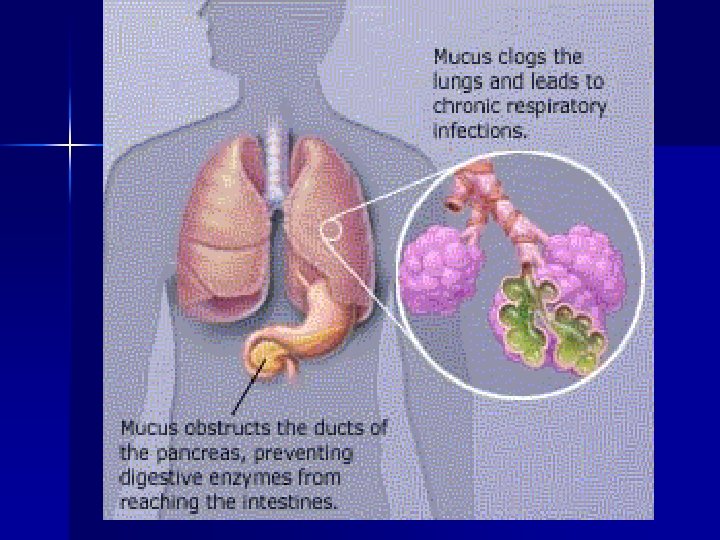
Cystic Fibrosis n n Epithelial cells and exocrine glands secrete abnormal mucus (thick) Affects respiratory tract, sweat, salivary, intestine, pancreas, liver, reproductive tract


Diagnosis of Cystic Fibrosis Neonatal screening provides opportunity to prevent malnutrition in CF infants n Sweat test (Na and Cl >60 m. Eq/L) n Chronic lung disease n Failure to thrive n Malabsorption n Family history n

Nutritional Implications of CF Infants born with meconium ileus are highly likely to have CF n 85% of persons with CF have pancreatic insufficiency n Plugs of mucus reduce the digestive enzymes released from the pancreas causing maldigestion of food and malabsorption of nutrients n

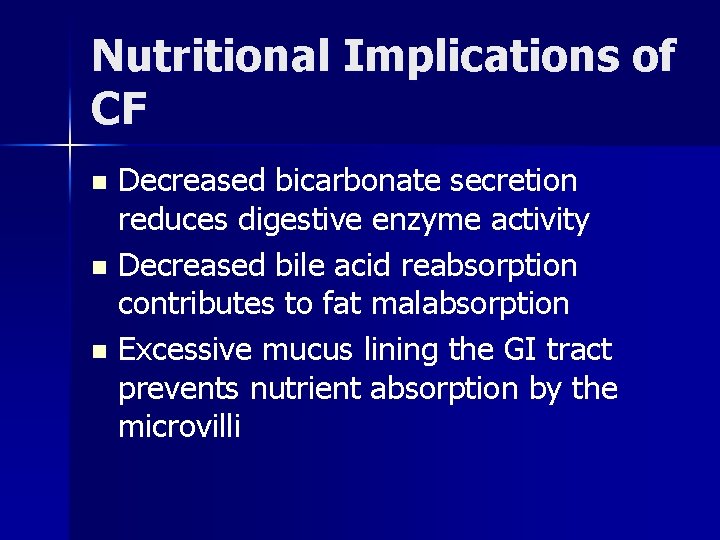
Nutritional Implications of CF Decreased bicarbonate secretion reduces digestive enzyme activity n Decreased bile acid reabsorption contributes to fat malabsorption n Excessive mucus lining the GI tract prevents nutrient absorption by the microvilli n

Gastrointestinal Complications of CF Bulky, foul-smelling stools n Cramping and intestinal obstruction n Rectal prolapse n Liver involvement n Pancreatic damage causes impaired glucose tolerance (50% of adults with CF) and development of diabetes (15% of adults with CF) n

Nutritional Care Goals Control malabsorption n Provide adequate nutrients for growth or maintain weight for height or pulmonary function n Prevent nutritional deficiencies n

Common Treatments Pancreatic enzyme replacement n Adjust macronutrients for symptoms n Nutrients for growth n n Meconium ileus equivalent: intestinal obstruction (enzymes, fiber, fluids, exercise, stool softeners)

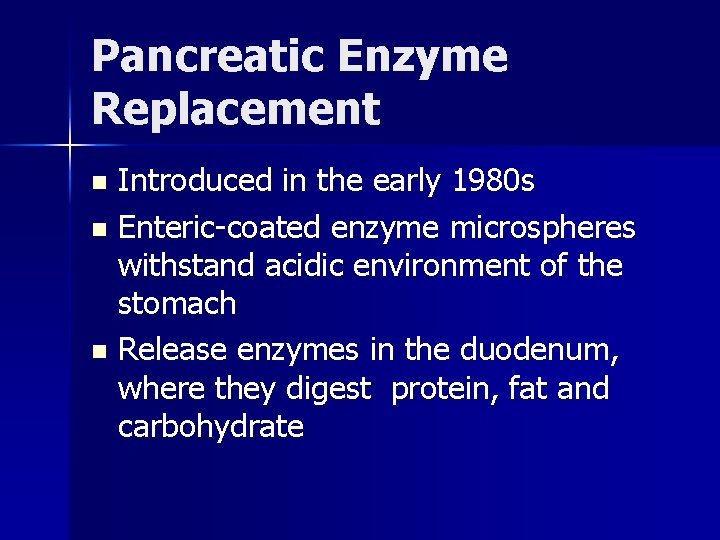
Pancreatic Enzyme Replacement Introduced in the early 1980 s n Enteric-coated enzyme microspheres withstand acidic environment of the stomach n Release enzymes in the duodenum, where they digest protein, fat and carbohydrate n

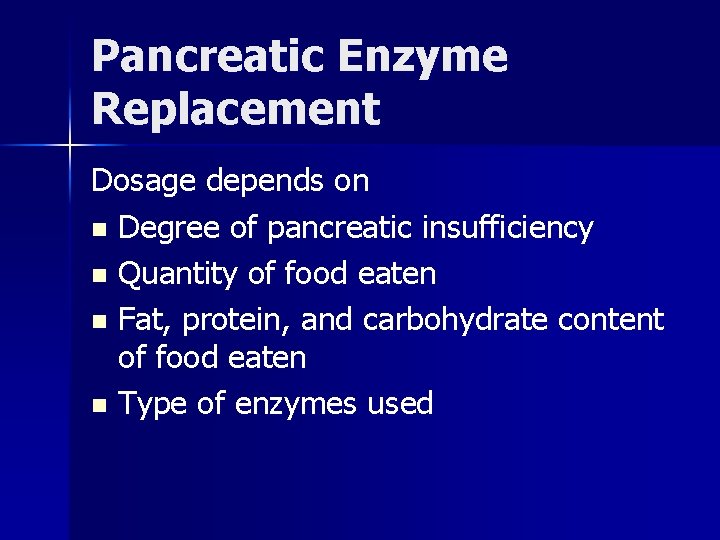
Pancreatic Enzyme Replacement Dosage depends on n Degree of pancreatic insufficiency n Quantity of food eaten n Fat, protein, and carbohydrate content of food eaten n Type of enzymes used

Pancreatic Enzyme Replacement n n n Enzyme dosage limited to 2500 lipase units per kilogram of body weight per meal Adjusted empirically to control gastrointestinal symptoms, including steatorrhea, and promote growth Fecal fat or nitrogen balance studies may help to evaluate the adequacy of enzyme supplementation

Distal Intestinal Obstruction Syndrome AKA recurrent intestinal impaction n Occurs in children and adults n Prevention includes adequate enzymes, fluids, dietary fiber, and regular exercise n Treatment involves stool softeners, laxatives, hyperosmolar enemas, intestinal lavage n

Estimation of Energy Needs in CF Use WHO equations to estimate BMR n Multiply by activity coefficient + disease coefficient n TEE – BMR X (AC + DC) n Disease coefficient is based on lung function n

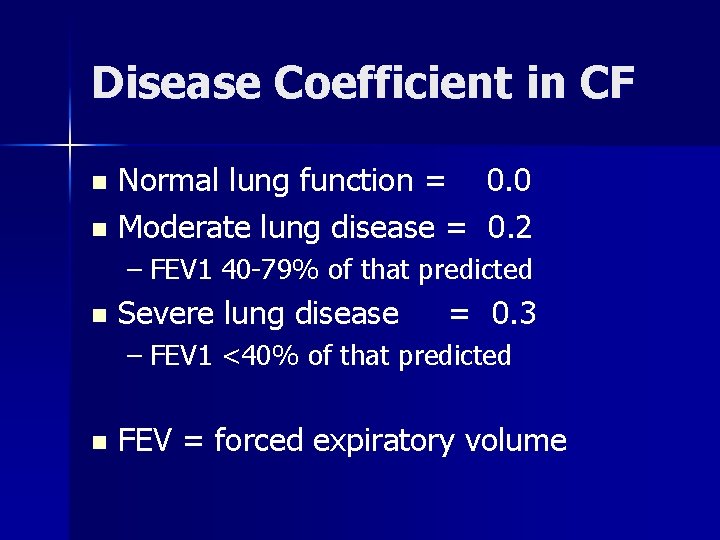
Disease Coefficient in CF Normal lung function = 0. 0 n Moderate lung disease = 0. 2 n – FEV 1 40 -79% of that predicted n Severe lung disease = 0. 3 – FEV 1 <40% of that predicted n FEV = forced expiratory volume

Example Equation TEE in CF Male patient 22 years old, weight 54 kg, relatively sedentary n FEV 1 is 60% of predicted (moderate lung disease) n TEE = BMR X (1. 5 + 0. 2) n TEE = [(15. 3 (54) + 679] X 1. 7 n TEE = 2559 kcals n

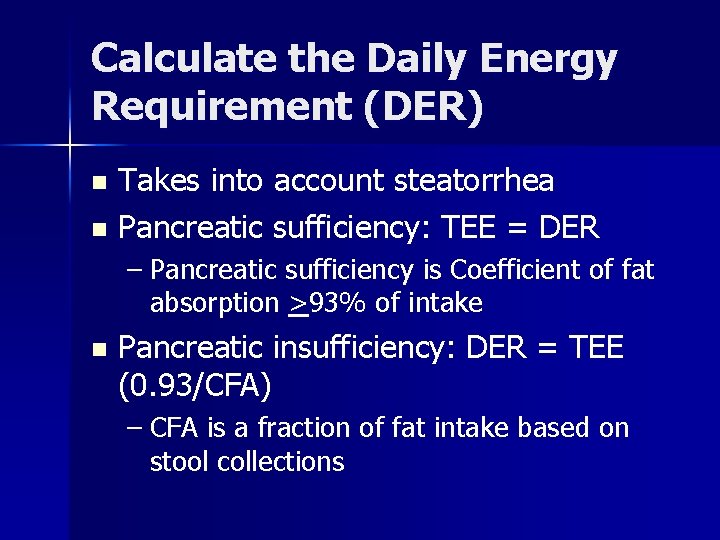
Calculate the Daily Energy Requirement (DER) Takes into account steatorrhea n Pancreatic sufficiency: TEE = DER n – Pancreatic sufficiency is Coefficient of fat absorption >93% of intake n Pancreatic insufficiency: DER = TEE (0. 93/CFA) – CFA is a fraction of fat intake based on stool collections

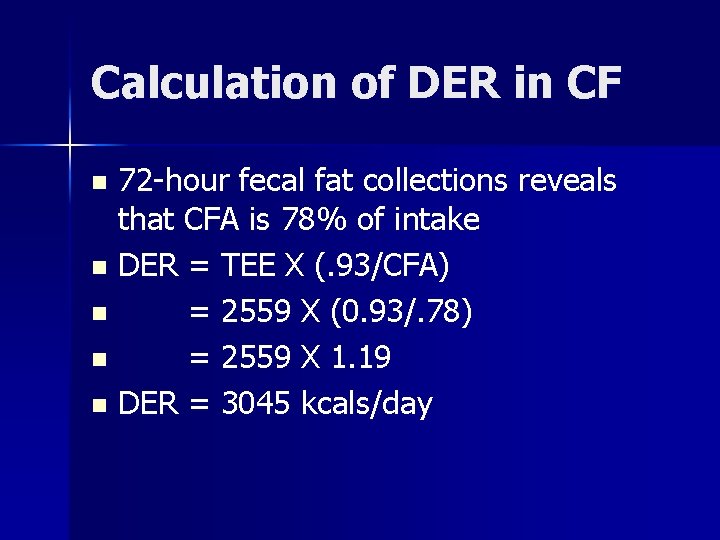
Calculation of DER in CF 72 -hour fecal fat collections reveals that CFA is 78% of intake n DER = TEE X (. 93/CFA) n = 2559 X (0. 93/. 78) n = 2559 X 1. 19 n DER = 3045 kcals/day n

Protein in CF Protein needs are increased in CF due to malabsorption n If energy needs are met, protein needs are usually met by following typical American diet (15 -20% protein) or use RDA n

Fat Intake in CF n n Fat intake 35 -40% of calories, as tolerated Helps provide required energy, essential fatty acids and fat-soluble vitamins Limits volume of food needed to meet energy demands and improves palatability of the diet EFA deficiency sometimes occurs in CF patients despite intake and pancreatic enzymes

Symptoms of Fat Intolerance Increased frequency of stools n Greasy stools n Abdominal cramping n

Carbohydrate in CF Eventually intake may need to be modified if glucose intolerance develops n Some patients develop lactose intolerance n

Vitamins in CF With pancreatic enzymes, water soluble vitamins usually adequately absorbed with daily multivitamin n Will need high potency supplementation of fat soluble vitamins (A, D, K, E) n

Minerals in CF Intake of minerals should meet DRI for age and sex n Sodium requirements increased due to loss in sweat n – North American diet usually provides enough – Infants need supplementation (1/4 -1/2 teaspoon/day)

Minerals in CF n Decreased bone mineralization, low iron stores, and low magnesium levels have all been described in CF

Feeding Strategies in CF: Infants Breast feeding with supplements of high-calorie formulas and pancreatic enzymes n Calorie dense infant formulas (20 -27 kcals/oz) with enzymes n Protein hydrolysate formulas with MCT oil if needed n

Feeding Strategies in CF: children and adults Regular mealtimes n Large portions n Extra snacks n Nutrient-dense foods n Nocturnal enteral feedings n – Intact or hydrolyzed formulas – Add enzyme powder to feeding or take before and during

Nutritional Implications of Tuberculosis n n TB is making a comeback Many patients are developing drugresistant TB

Nutritional Factors that Increase Risk of TB Protein-energy malnutrition: affects the immune system; debate whether it is a cause or consequence of the disease n Micronutrient deficiencies that affect immune function (vitamin D, A, C, iron, zinc) n

Nutritional Consequences of TB Increased energy expenditure n Loss of appetite and body weight n Increase in protein catabolism leading to muscle breakdown n Malabsorption causing diarrhea, loss of fluids, electrolytes n

Nutritional Needs in TB Energy: 35 -40 kcals/kg of ideal body weight n Protein: 1. 2 -1. 5 grams/kg body weight, or 15% of energy or 75 -100 grams/day n Multivitamin-mineral supplement at 100 -150% DRI n

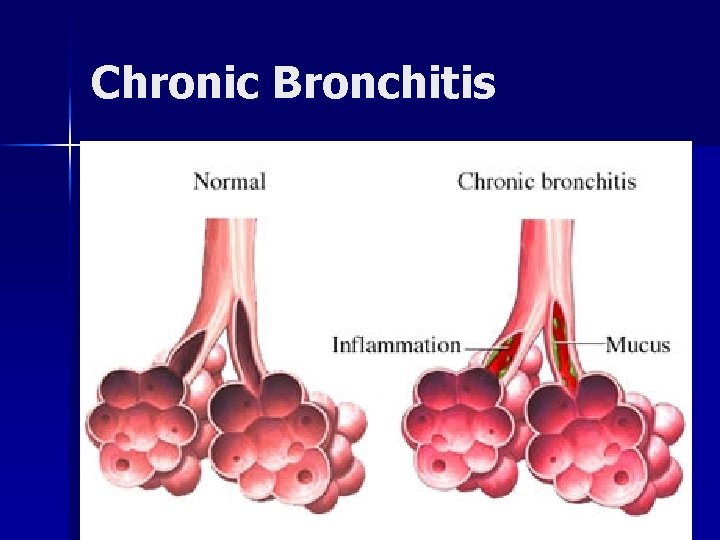
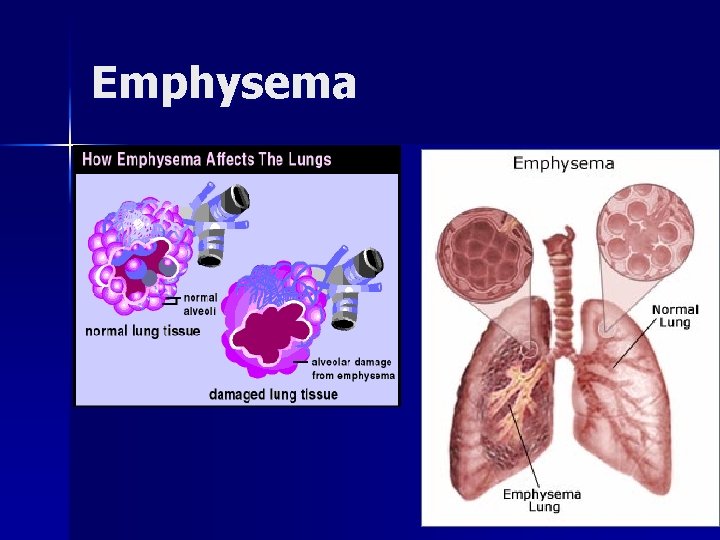
Chronic Obstructive Pulmonary Disease (COPD) Characterized by airway obstruction n Emphysema: abnormal, permanent enlargement of alveoli, accompanied by destruction of their walls without obvious fibrosis n Chronic bronchitis: chronic, productive cough with inflammation of one or more of the bronchi and secondary changes in lung tissue

Chronic Obstructive Pulmonary Disease (COPD) Emphysema: patients are thin, often cachectic; older, mild hypoxia, normal hematocrits n Chronic bronchitis: of normal weight; often overweight; hypoxia; high hematocrit n

Chronic Obstructive Pulmonary Disease (COPD) Bronchospasm: asthma n Cor pumonale: heart condition characterized by right ventricular enlargement and failure that results from resistance to passage of blood through the lungs n

Chronic Bronchitis

Emphysema

Bronchial Asthma Food sensitivities may be triggers for asthmatic episodes (sulfites, shrimp, herbs) but not the most common causes n Provide healthy diet and maintain healthy weight n Be aware of drug nutrient interactions (steroids) n


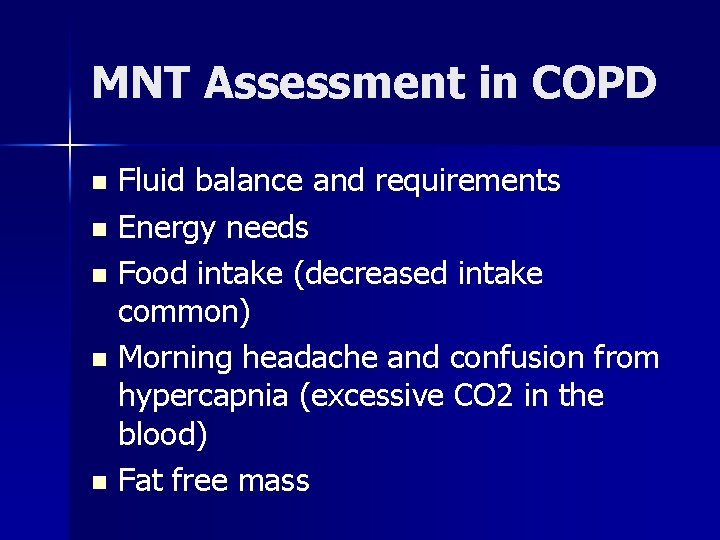
MNT Assessment in COPD Fluid balance and requirements n Energy needs n Food intake (decreased intake common) n Morning headache and confusion from hypercapnia (excessive CO 2 in the blood) n Fat free mass n

MNT Assessment in COPD n n n Food drug interactions Fatigue Anorexia Difficulty chewing/swallowing because of dyspnea Impaired peristalsis secondary to lack of oxygen to the GI tract Underweight patients have the highest morbidity/mortality

Nutrient Needs in Stable COPD n n n Protein: 1. 2 -1. 7 grams/kg (15 -20% of calories) to restore lung and muscle strength and promote immune function Fat: 30 -45% of calories Carbohydrate: 40 -55% of calories Maintain appropriate RQ Address other underlying diseases (diabetes, heart disease)

Nutrient Needs in Stable COPD n n n Vitamins: intakes should at least meet the DRI Smokers may need more vitamin C (+16 -32 mg) depending on cigarette use Minerals: meet DRIs and monitor phosphorus and magnesium in patients at risk for refeeding during aggressive nutrition support

Treatments for COPD Bronchodilators—theophylline and aminophylline n Antibiotics—secondary infections n Respiratory therapy n Exercise to strengthen muscles n

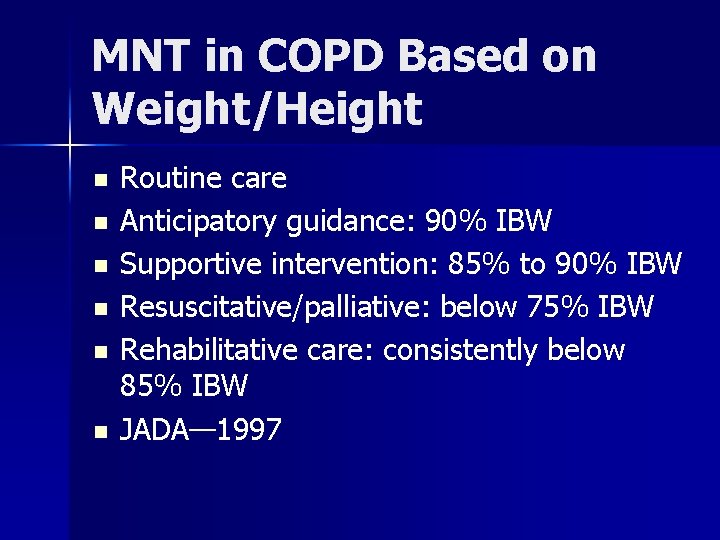
MNT in COPD Based on Weight/Height n n n Routine care Anticipatory guidance: 90% IBW Supportive intervention: 85% to 90% IBW Resuscitative/palliative: below 75% IBW Rehabilitative care: consistently below 85% IBW JADA— 1997

MNT in COPD GI motility: adequate exercise, fluids, dietary fiber n Abdominal bloating: limit foods associated with gas formation n Fatigue: resting before meals, eating nutrient-dense foods, arrange assistance with shopping and meal preparation n

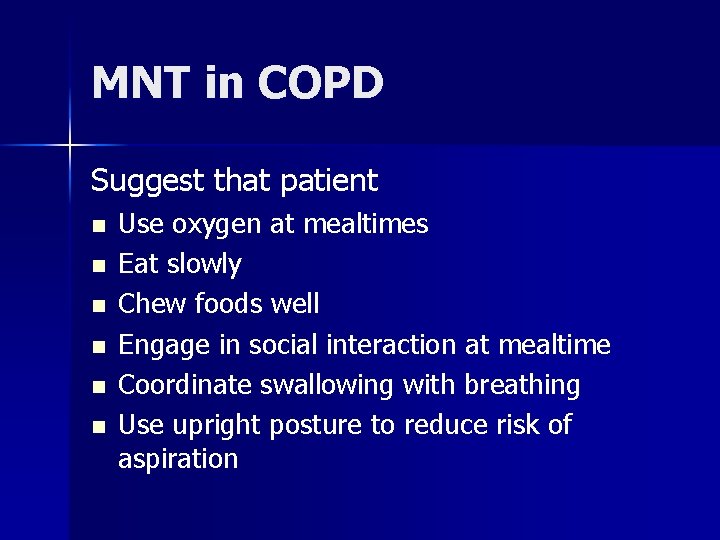
MNT in COPD Suggest that patient n n n Use oxygen at mealtimes Eat slowly Chew foods well Engage in social interaction at mealtime Coordinate swallowing with breathing Use upright posture to reduce risk of aspiration

MNT in COPD Oral supplements n Nocturnal or supplemental tube feedings n Specialized pulmonary products generally not necessary n

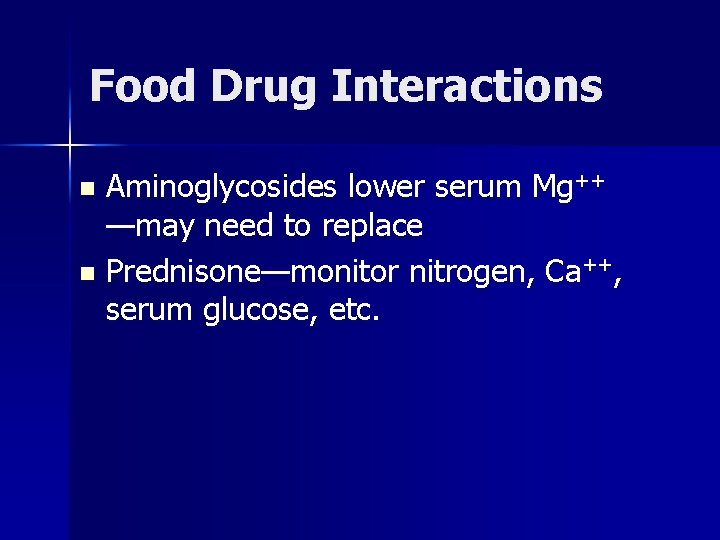
Food Drug Interactions Aminoglycosides lower serum Mg++ —may need to replace n Prednisone—monitor nitrogen, Ca++, serum glucose, etc. n

MNT in Respiratory Failure

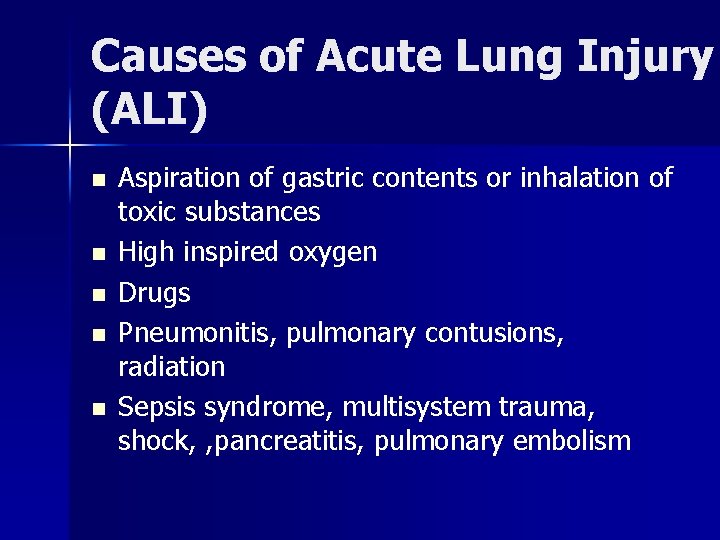
Causes of Acute Lung Injury (ALI) n n n Aspiration of gastric contents or inhalation of toxic substances High inspired oxygen Drugs Pneumonitis, pulmonary contusions, radiation Sepsis syndrome, multisystem trauma, shock, , pancreatitis, pulmonary embolism

Aspiration Movement of food or fluid into the lungs n Can result in pneumonia or even death n Increased risk for infants, toddlers, older adults, persons with oral, upper gastrointestinal, neurologic, or muscular abnormalities n

Aspiration n n Reported incidence of aspiration in tubefed patients varies from. 8% to 95%. Clinically significant aspiration 1 -4% Many aspiration events are “silent” and often involve oropharyngeal secretions Symptoms include dyspnea, tachycardia, wheezing, rales, anxiety, agitation, cyanosis May lead to aspiration pneumonia

Acute Respiratory Distress Syndrome (ARDS) Most severe form of acute lung injury n Sepsis usually the underlying cause n Increasing pulmonary capillary permeability n Pulmonary edema n Increased pulmonary vascular resistance n Progressive hypoxemia n

Goals of Treatment of ALI and ARDS Improve oxygen delivery and provide hemodynamic support n Reduce oxygen consumption n Optimize gas exchange n Individualize nutrition support n

Nutrition Assessment in ALI and ARDS Indirect calorimetry best tool to determine energy needs in critically ill patients n In absence of calorimetry, use predictive equations with stress factors n Avoid overfeeding n Patients may need high calorie density feedings to achieve fluid balance n

Nutrition Support in ARDS n n In one randomized, controlled trial in 146 patients with ARDS, enteral nutrition with omega-3 fatty acids (eicosapentaenoic acid) gamma-linonenic acid, and antioxidants appeared to reduce days on mechanical ventilation, new organ failure, and ICU length of stay This study was sponsored by Ross Laboratories, makers of Oxepa Have been unable to locate further studies since then Gadek JE et al. Effect of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in patients with acute respiratory distress syndrome. Enteral Nutrition in ARDS Study Group. Crit Care Med 1999; 27: 1409.
 Medical nutrition therapy for stroke
Medical nutrition therapy for stroke Medical nutrition therapy for hypertension
Medical nutrition therapy for hypertension Small bowel obstruction nutrition management
Small bowel obstruction nutrition management Pulmonary disease definition
Pulmonary disease definition Copd full form in medical
Copd full form in medical Nutrition fundamentals of nursing
Nutrition fundamentals of nursing Communicable disease and non communicable disease
Communicable disease and non communicable disease Stomach ulcer
Stomach ulcer Pud triple therapy
Pud triple therapy Triple therapy for peptic ulcer disease
Triple therapy for peptic ulcer disease Gene therapy for sickle cell disease
Gene therapy for sickle cell disease Psychoanalytic vs humanistic
Psychoanalytic vs humanistic Bioness integrated therapy system price
Bioness integrated therapy system price Humanistic therapy aims to
Humanistic therapy aims to Maternal and child malnutrition
Maternal and child malnutrition Medical family therapy
Medical family therapy Difference between medical report and medical certificate
Difference between medical report and medical certificate What is the physiology of respiration
What is the physiology of respiration Pulmonary artery and aorta
Pulmonary artery and aorta Bronchioles
Bronchioles Pulmonary ventilation consists of two cyclic phases, , and
Pulmonary ventilation consists of two cyclic phases, , and Aorta inferior vena cava
Aorta inferior vena cava Aorta and pulmonary artery
Aorta and pulmonary artery Parts of the heart
Parts of the heart Circulatory system diagram
Circulatory system diagram Pulmonary volumes and capacities
Pulmonary volumes and capacities Unicef conceptual framework
Unicef conceptual framework Hypothemi
Hypothemi Definition of protein-energy malnutrition
Definition of protein-energy malnutrition Moderate acute malnutrition
Moderate acute malnutrition Kanawati index
Kanawati index Holliday segar calories
Holliday segar calories Malnutrition definition
Malnutrition definition Malnutrition
Malnutrition Conclusion for malnutrition
Conclusion for malnutrition Definition malnutrition
Definition malnutrition Z score for malnutrition
Z score for malnutrition Icd-10 malnutrition anak
Icd-10 malnutrition anak Iatrogenic malnutrition
Iatrogenic malnutrition What is the difference between kwashiorkor and marasmus
What is the difference between kwashiorkor and marasmus Malnutrition conceptual framework
Malnutrition conceptual framework Malnutrition case presentation
Malnutrition case presentation Resomal
Resomal Aspen criteria for malnutrition
Aspen criteria for malnutrition Malnutrition
Malnutrition Nutrition fundamentals of nursing
Nutrition fundamentals of nursing Fluid of choice in severe dehydration
Fluid of choice in severe dehydration Chief complaint of colostomy
Chief complaint of colostomy Marasmus vs kwashiorkor
Marasmus vs kwashiorkor Stunting
Stunting Malnutrition
Malnutrition Conclusion of malnutrition in india
Conclusion of malnutrition in india Malnutrition definition
Malnutrition definition Malnutrition
Malnutrition Malnutrition quality improvement initiative
Malnutrition quality improvement initiative Malnutrition conclusion
Malnutrition conclusion Malnutrition conclusion
Malnutrition conclusion Nursing diagnosis of marasmus
Nursing diagnosis of marasmus Balance diet conclusion
Balance diet conclusion Malnutrition flowchart
Malnutrition flowchart Prevention of malnutrition
Prevention of malnutrition 30 / 4
30 / 4 Malnutrition physical exam
Malnutrition physical exam Ptal california medical board
Ptal california medical board Gbmc medical records
Gbmc medical records Torrance memorial hospital medical records
Torrance memorial hospital medical records Cartersville medical center medical records
Cartersville medical center medical records Tapvc
Tapvc Thoracic cavity
Thoracic cavity Systemic circulation
Systemic circulation Neurogenic shock pathophysiology
Neurogenic shock pathophysiology Heart
Heart Blood gas
Blood gas Pulmonary surfactant function
Pulmonary surfactant function Pulmonary embolism cxr
Pulmonary embolism cxr Flow volume loop obstructive
Flow volume loop obstructive Hampton hump xray
Hampton hump xray Pulmonary embolism diagnosis
Pulmonary embolism diagnosis Pah vs pulmonary hypertension
Pah vs pulmonary hypertension Light criteria
Light criteria Pleural adhesions
Pleural adhesions Muscles of inspiration and expiration
Muscles of inspiration and expiration Svr calculation
Svr calculation Pulmonary toilet
Pulmonary toilet Pulmonary edema
Pulmonary edema Papillary muscles sheep heart
Papillary muscles sheep heart Pulmonary toilet
Pulmonary toilet