MEDICAL IMPLICATIONS OF DEVELOPMENTAL BIOLOGY IVF In vitro

MEDICAL IMPLICATIONS OF DEVELOPMENTAL BIOLOGY
IVF


• In vitro fertilization (IVF) is a complex series of procedures used to help with fertility or prevent genetic problems and assist with the conception of a child • IVF is the most effective form of assisted reproductive technology. • IVF is performed to treat infertility • IVF combines the egg and sperm in a laboratory

Reasons for IVF • Fallopian tube damage or blockage makes it difficult for an egg to be fertilized or for an embryo to travel to the uterus. • Ovulation disorders. If ovulation is infrequent or absent, fewer eggs are available for fertilization. • Endometriosis occurs when the uterine tissue implants and grows outside of the uterus — often affecting the function of the ovaries, uterus and fallopian tubes. • A genetic disorder

• Uterine fibroids. Fibroids are benign tumors in the wall of the uterus and are common in women in their 30 s and 40 s. Fibroids can interfere with implantation of the fertilized egg. • Previous tubal sterilization or removal. If you've had tubal ligation — a type of sterilization in which your fallopian tubes are cut or blocked to permanently prevent pregnancy — and want to conceive, IVF may be an alternative to tubal ligation reversal. • Impaired sperm production or function. Below-average sperm concentration, weak movement of sperm (poor mobility), or abnormalities in sperm size and shape can make it difficult for sperm to fertilize an egg. If semen abnormalities are found, your partner might need to see a specialist to determine if there are correctable problems or underlying health concerns. Fertility preservation for cancer or other health conditions

PATIENT PREPARATION • Ovarian reserve testing. To determine the quantity and quality of your eggs, the doctor might test the concentration of folliclestimulating hormone (FSH), estradiol (estrogen) and anti-mullerian hormone in blood during the first few days of menstrual cycle. • Test results, often used together with an ultrasound of ovaries, can help predict how your ovaries will respond to fertility medication.
• Semen analysis.

• Infectious disease screening. Both the partners be screened for infectious diseases, including HIV. • Uterine exam. Doctor will examine the inside lining of the uterus before the start of IVF. Sonohysterography — in which fluid is injected through the cervix into the uterus — and an ultrasound to create images of uterine cavity. Hysteroscopy — in which a thin, flexible, lighted telescope (hysteroscope) is inserted through vagina and cervix into uterus.

• How many embryos will be transferred? The number of embryos transferred is based on age and number of eggs retrieved. • Since the rate of implantation is lower for older women, more embryos are usually transferred • Most doctors follow specific guidelines to prevent a higher order multiple pregnancy — triplets or more. • The doctor and patient agree on the number of embryos that will be transferred before the transfer procedure.

• extra embryos: Extra embryos can be frozen and stored for future use for several years. Not all embryos will survive the freezing and thawing process. • Donate unused frozen embryos to another couple or a research facility. • Patient can choose to discard unused embryos.

multiple pregnancy: • If more than one embryo is transferred IVF can result in a multiple pregnancy — which poses health risks for you and your babies. • In some cases, fetal reduction can be used to help a woman deliver fewer babies with lower health risks. Pursuing fetal reduction, however, is a major decision with ethical, emotional and psychological consequences.
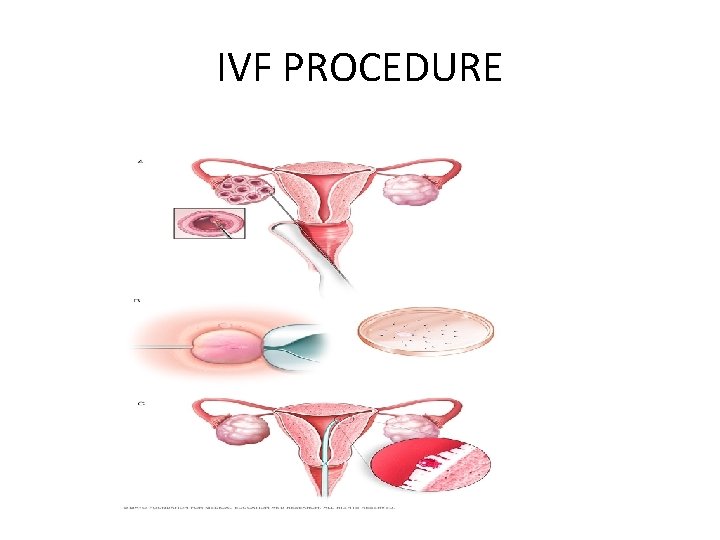
IVF PROCEDURE

• IVF involves several steps — • ovarian stimulation • egg retrieval • sperm retrieval • fertilization and embryo transfer. One cycle of IVF can take about two to three weeks, and more than one cycle may be required.

• Ovulation induction-needs one to two weeks • Synthetic hormones to stimulate ovaries to produce multiple eggs — rather than the single egg that normally develops each month. Multiple eggs are needed because some eggs won't fertilize or develop normally after fertilization • Medications for ovarian stimulation. Injectable medication containing a follicle-stimulating hormone (FSH), a luteinizing hormone (LH) or a combination of both. These medications stimulate more than one egg to develop at a time. • Medications for oocyte maturation. When the follicles are ready for egg retrieval — generally after eight to 14 days — human chorionic gonadotropin (HCG) or other medications to help the eggs mature. • Medications to prevent premature ovulation. These medications prevent the body from releasing the developing eggs too soon. • Medications to prepare the lining of uterus. On the day of egg retrieval or at the time of embryo transfer, begin to take progesterone supplements to make the lining of your uterus more receptive to implantation.

EGG RETRIEVAL • Vaginal ultrasound, an imaging exam of ovaries to monitor the development of follicles — fluid-filled ovarian sacs where eggs mature • Blood tests, to measure the response to ovarian stimulation medications — estrogen levels typically increase as follicles develop, and progesterone levels remain low until after ovulation Sometimes IVF cycles need to be canceled before egg retrieval for one of these reasons: • Inadequate number of follicles developing • Premature ovulation • Other medical issues • If your cycle is canceled, the doctor might recommend changing medications or their doses to promote a better response during future IVF cycles. Or you may be advised that you need an egg donor

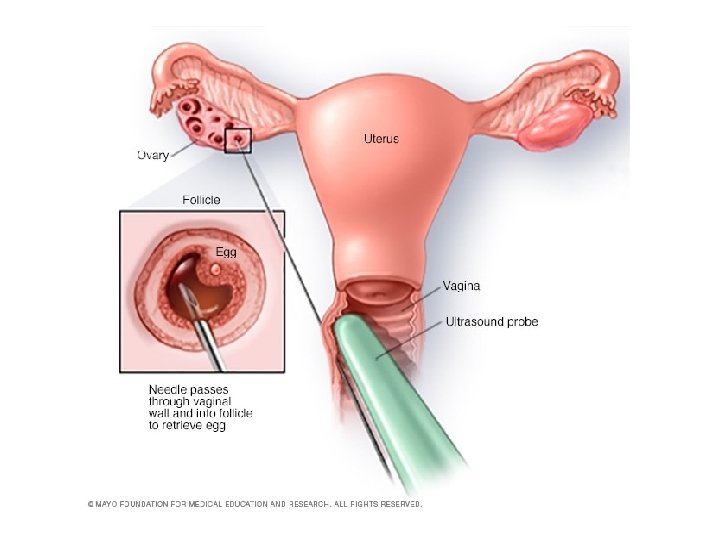
• Egg retrieval can be done in doctor's clinic 34 to 36 hours after the final injection and before ovulation. • During egg retrieval, patient 'll be sedated and given pain medication. • Transvaginal ultrasound aspiration is the usual retrieval method. An ultrasound probe is inserted into your vagina to identify follicles. Then a thin needle is inserted into an ultrasound guide to go through the vagina and into the follicles to retrieve the eggs. • If ovaries aren't accessible through transvaginal ultrasound, an abdominal ultrasound may be used to guide the needle. • The eggs are removed from the follicles through a needle connected to a suction device. Multiple eggs can be removed in about 20 minutes. • After egg retrieval, you may experience cramping and feelings of fullness or pressure. • Mature eggs are placed in a nutritive liquid (culture medium) and incubated. Eggs that appear healthy and mature will be mixed with sperm to attempt to create embryos. However, not all eggs may be successfully fertilized.

• Sperm retrieval semen sample at a clinic through masturbation the morning of egg retrieval. Other methods, such as testicular aspiration — the use of a needle or surgical procedure to extract sperm directly from the testicle — are sometimes required. Donor sperm also can be used. Sperm are separated from the semen fluid in the lab.

• Fertilization can be attempted using two common methods: • Conventional insemination. During conventional insemination, healthy sperm and mature eggs are mixed and incubated overnight. • Intracytoplasmic sperm injection (ICSI). In ICSI, a single healthy sperm is injected directly into each mature egg. ICSI is often used when semen quality or number is a problem or if fertilization attempts during prior IVF cycles failed.

ICSI • The mature egg is held with a specialized pipette. • A very delicate, sharp, and hollow needle is used to immobilize and pick up a single sperm. • The needle is then carefully inserted through the shell of the egg and into the cytoplasm of the egg. • The sperm is injected into the cytoplasm, and the needle is carefully removed. • The eggs are checked the following day for evidence of normal fertilization.
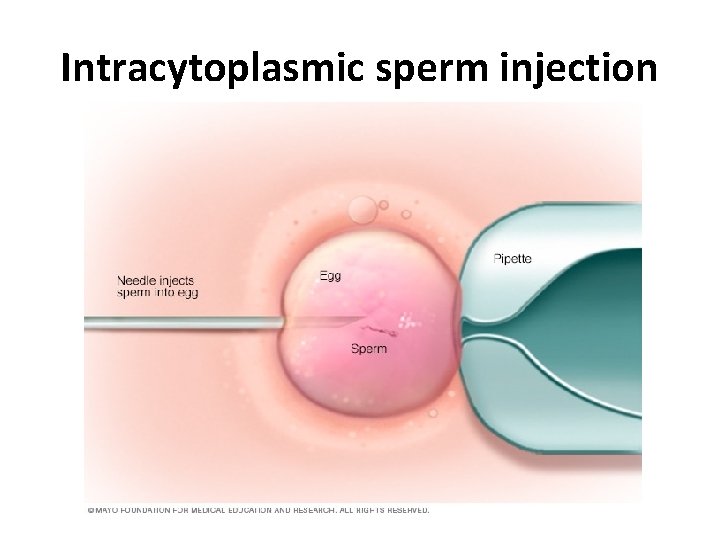
Intracytoplasmic sperm injection
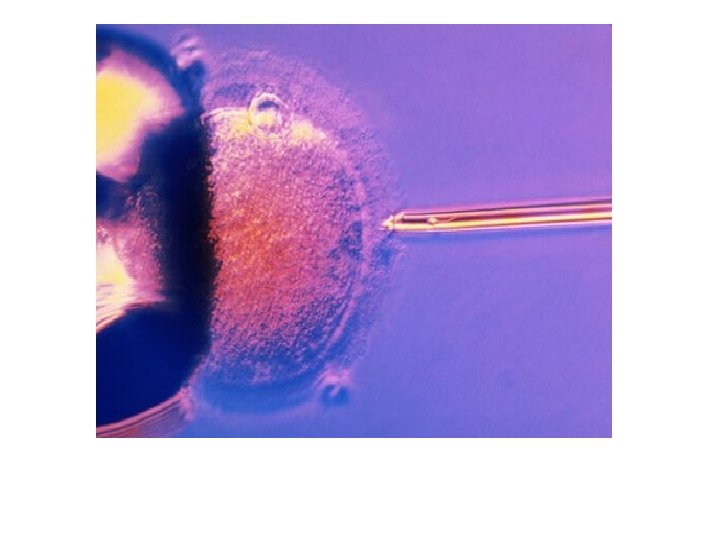

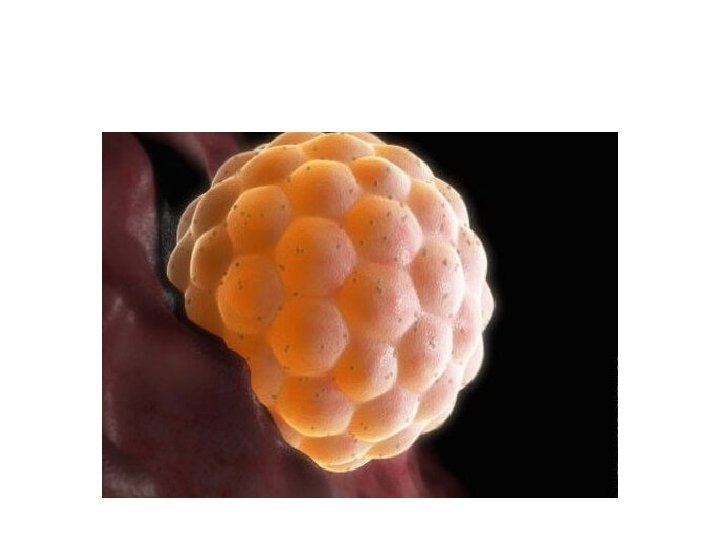
• Assisted hatching. About five to six days after fertilization, an embryo "hatches" from its surrounding membrane (zona pellucida), allowing it to implant into the lining of the uterus. If an older woman, or if had multiple failed IVF attempts, your doctor might recommend assisted hatching — a technique in which a hole is made in the zona pellucida just before transfer to help the embryo hatch and implant. • Assisted hatching is also useful for eggs or embryos that have been previously frozen as the process can harden the zona pellucida.
• Preimplantation genetic testing. Embryos are allowed to develop in the incubator until they reach a stage where a small sample can be removed and tested for specific genetic diseases or the correct number of chromosomes, typically after five to six days of development. Embryos that don't contain affected genes or chromosomes can be transferred to your uterus. While preimplantation genetic testing can reduce the likelihood that a parent will pass on a genetic problem, it can't eliminate the risk. Prenatal testing may still be recommended.
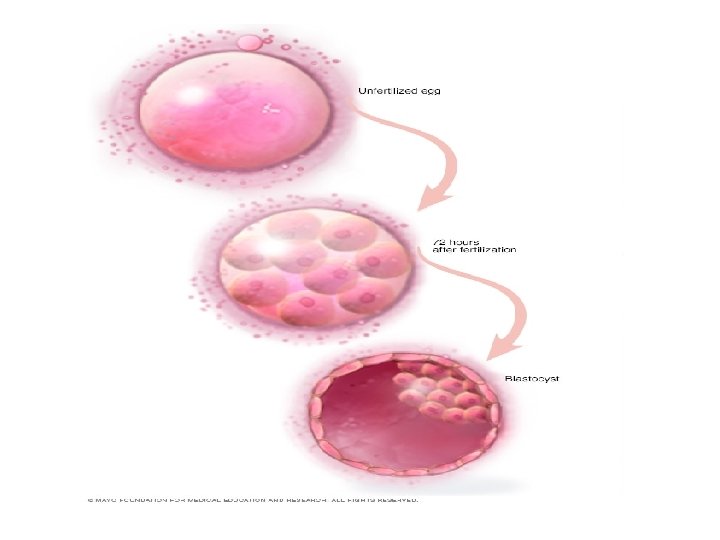
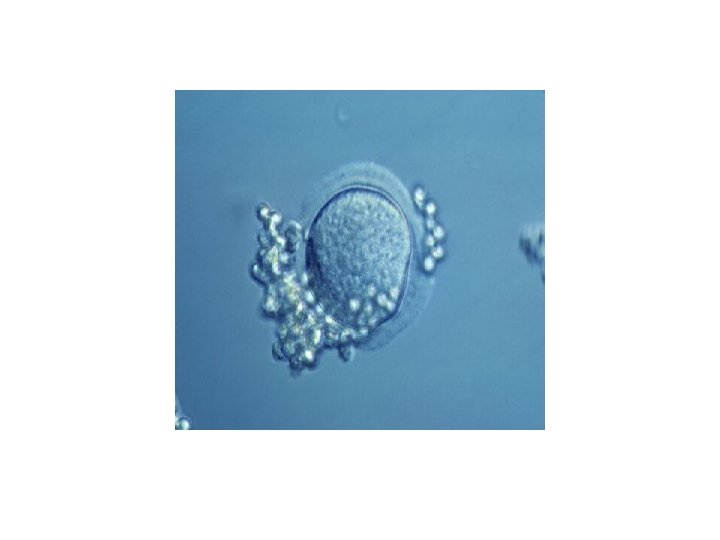

Embryo transfer • Embryo transfer is done at your doctor's clinic and usually takes place two to five days after egg retrieval. • Patient was given a mild sedative. The procedure is usually painless, although it might experience mild cramping. • The doctor will insert a long, thin, flexible tube called a catheter into the vagina, through cervix and into uterus. • A syringe containing one or more embryos suspended in a small amount of fluid is attached to the end of the catheter. • Using the syringe, the doctor places the embryo or embryos into uterus. • If successful, an embryo will implant in the lining of uterus about six to 10 days after egg retrieval.

Hormone regulation • The luteal phase of the cycle, and the woman is given the hormone progesterone, either as injections or vaginal suppositories. Sometimes progesterone in both forms will be given. Progesterone administration continues for the next 2 weeks. A pregnancy test is scheduled for two weeks following the embryo transfer. If implantation is successful (the egg or eggs attach to the uterine wall and grow), the pregnancy test result should be positive
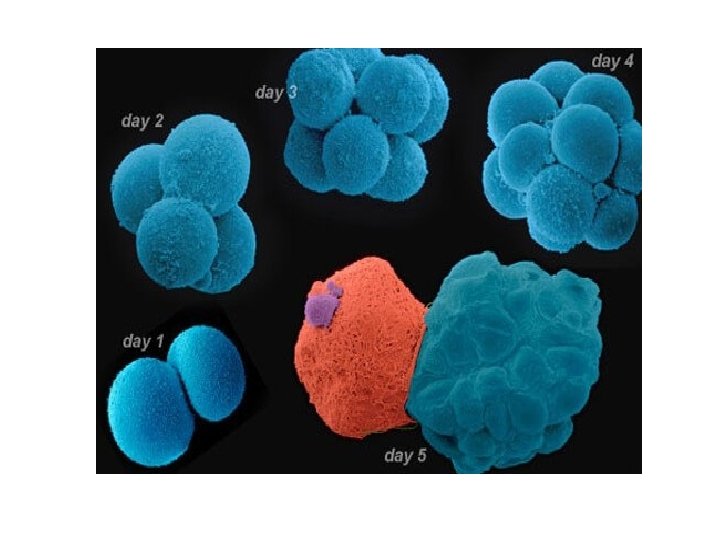
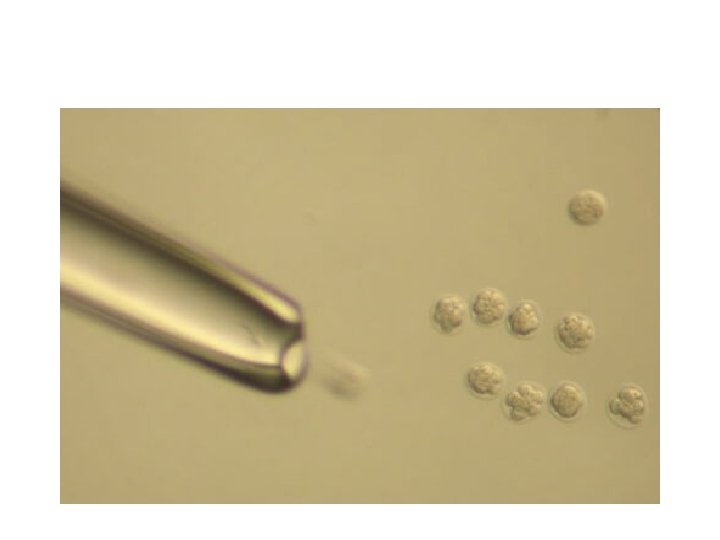

After the procedure • After the embryo transfer, you can resume normal daily activities. However, your ovaries may still be enlarged. Consider avoiding vigorous activity, which could cause discomfort. • Typical side effects include: • Passing a small amount of clear or bloody fluid shortly after the procedure — due to the swabbing of the cervix before the embryo transfer • Breast tenderness due to high estrogen levels • Mild bloating • Mild cramping • Constipation • If you develop moderate or severe pain after the embryo transfer, contact your doctor. He or she will evaluate you for complications such as infection, twisting of an ovary (ovarian torsion) and severe ovarian hyperstimulation syndrome.

• RESULTS • About 12 days to two weeks after egg retrieval, doctor will test a sample of blood to detect whether you're pregnant. • If you're pregnant, doctor will refer to an obstetrician or other pregnancy specialist for prenatal care. • If you're not pregnant, you'll stop taking progesterone and likely get your period within a week. If you don't get your period or you have unusual bleeding, contact your doctor. If you're interested in attempting another cycle of in vitro fertilization (IVF), your doctor might suggest steps you can take to improve your chances of getting pregnant through IVF.

Healthy baby after using IVF depend on various factors • Maternal age. The younger you are, the more likely you are to get pregnant and give birth to a healthy baby using your own eggs during IVF. Women age 41 and older are often counseled to consider using donor eggs during IVF to increase the chances of success. • Embryo status. Transfer of embryos that are more developed is associated with higher pregnancy rates compared with less-developed embryos (day two or three). However, not all embryos survive the development process.

• Reproductive history. Women who've previously given birth are more likely to be able to get pregnant using IVF than are women who've never given birth. Success rates are lower for women who've previously used IVF multiple times but didn't get pregnant. • Cause of infertility. Having a normal supply of eggs increases the chances of being able to get pregnant using IVF. Women who have severe endometriosis are less likely to be able to get pregnant using IVF than are women who have unexplained infertility. • Lifestyle factors. Women who smoke typically have fewer eggs retrieved during IVF and may miscarry more often. Smoking can lower a woman's chance of success using IVF by 50%. Obesity can decrease the chances of getting pregnant and having a baby. Use of alcohol, recreational drugs, excessive caffeine and certain medications also can be harmful.

Success rates • The live birth rate per IVF cycle is 54% among women younger than 35 years of age and 42% for those aged 35 to 37 years. • The success rate ranges from 3. 9% to 13. 3% in those older than 40 years of age. • Pregnancy in women older than 44 years of age is rare.

Risks • Risks of IVF include: • Multiple births. IVF increases the risk of multiple births if more than one embryo is transferred to uterus. A pregnancy with multiple fetuses carries a higher risk of early labor and low birth weight than pregnancy with a single fetus does. • Premature delivery and low birth weight. IVF slightly increases the risk that the baby will be born early or with a low birth weight.
Multiple

• Ovarian hyperstimulation syndrome. Use of injectable fertility drugs, such as human chorionic gonadotropin (HCG), to induce ovulation cause ovarian hyperstimulation syndrome, in which your ovaries become swollen and painful. • Symptoms typically last a week and include mild abdominal pain, bloating, nausea, vomiting and diarrhea. Can also cause rapid weight gain and shortness of breath.
• Miscarriage. The rate of miscarriage for women who conceive using IVF with fresh embryos is similar to that of women who conceive naturally — about 15% to 25% — but the rate increases with maternal age. • Egg-retrieval procedure complications. Use of an aspirating needle to collect eggs could possibly cause bleeding, infection or damage to the bowel, bladder or a blood vessel. Risks are also associated with sedation and general anesthesia, if used.

• Ectopic pregnancy. About 2% to 5% of women who use IVF will have an ectopic pregnancy — when the fertilized egg implants outside the uterus, usually in a fallopian tube. The fertilized egg can't survive outside the uterus, and there's no way to continue the pregnancy. • Birth defects. The age of the mother is the primary risk factor in the development of birth defects, no matter how the child is conceived. More research is needed to determine whether babies conceived using IVF might be at increased risk of certain birth defects.

• Cancer. Although some early studies suggested there may be a link between certain medications used to stimulate egg growth and the development of a specific type of ovarian tumor, more-recent studies do not support these findings. There does not appear to be a significantly increased risk of breast, endometrial, cervical or ovarian cancer after IVF. • Stress. Use of IVF can be financially, physically and emotionally draining. Support from counselors, family and friends can help you and your partner through the ups and downs of infertility treatment.

ART • Gamete intrafallopian transfer (GIFT): Gamete intrafallopian transfer is similar to IVF. It is used when a woman has at least one normal Fallopian tube. Eggs are placed in this tube along with a man's sperm to fertilize there. This accounts for only a small portion of assisted reproductive technology procedures in the US. Some couples opt for this procedure if they object to fertilization that occurs outside the woman's body.
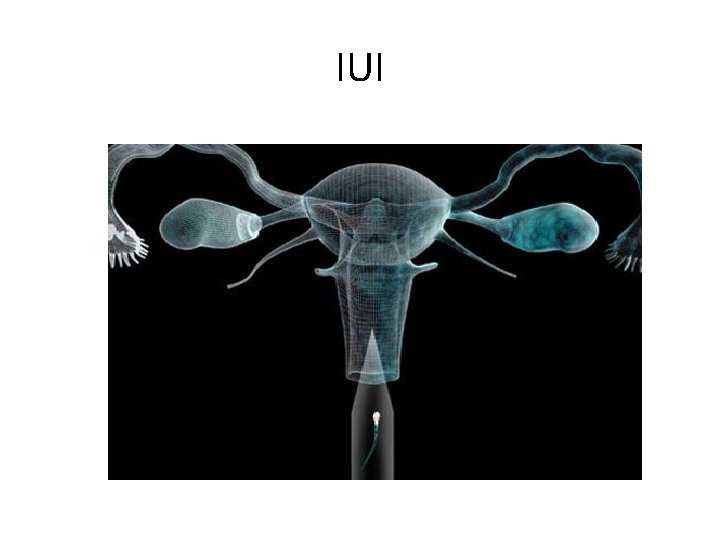
IUI

• Zygote intrafallopian transfer (ZIFT): Zygote intrafallopian transfer refers to a procedure in which a woman's eggs are taken from her ovaries, fertilized in the laboratory, and inserted into her Fallopian tubes rather than the uterus. ZIFT is even less common than GIFT.

• Embryo cryopreservation (frozen fertilized egg and sperm) is available when more embryos are created than are transferred to the woman's uterus. These can be transferred during a future cycle. In this case a woman would take medications to prepare her uterus to receive the embryos at the appropriate time.


Gene therapy • Altering the genes inside your body's cells in an effort to treat or stop disease. • DNA — the code that controls much of your body's form and function, from making you grow taller to regulating your body systems. • Genes that don't work properly can cause disease.

• Gene therapy replaces a faulty gene • Adds a new gene in an attempt to cure disease or improve body's ability to fight disease. • Gene therapy holds promise for treating a wide range of diseases, such as cancer, cystic fibrosis, heart disease, diabetes, hemophilia and AIDS. • Researchers are still studying how and when to use gene therapy. • Currently, in the United States, gene therapy is available only as part of a clinical trial.

Replacing mutated genes. Replacing the defective genes may help treat certain diseases. For instance, a gene called p 53 normally prevents tumor growth. Several types of cancer have been linked to problems with the p 53 gene. If doctors could replace the defective p 53 gene, that might trigger the cancer cells to die. • Fixing mutated genes. Mutated genes that cause disease could be turned off so that they no longer promote disease, or healthy genes that help prevent disease could be turned on so that they could inhibit the disease.

• Making diseased cells more evident to the immune system. Some cases, immune system doesn't attack diseased cells because it doesn't recognize them as intruders. • Gene therapy to train immune system to recognize the cells that are a threat.
Clinical Trials • The blood or bone marrow are exposed to a virus or another type of vector that contains the desired genetic material. • Once the vector has entered the cells in the lab, those cells are injected back into your body into a vein or into tissue, where your cells take up the vector along with the altered genes. • Viruses aren't the only vectors that can be used to carry altered genes into your body's cells.
• Viruses have evolved a way of encapsulating and delivering their genes to human cells in a pathogenic manner. • Scientists have tried this ability by manipulating the viral genome to remove disease-causing genes and insert therapeutic ones. • Target cells such as the patient's liver or lung cells are infected with the vector. • The vector then unloads its genetic material containing therapeutic human gene into the target cell. • The generation of a functional protein product from therapeutic gene restores the target cell to a normal state. • In theory it is possible to transform either somatic cells (most cells of the body) or cells of the germline (such as sperm cells, ova, and their stem cell precursors). • All gene therapy to date on humans has been directed at somatic cells, whereas germline engineering in humans remains controversial. • For the introduced gene to be transmitted normally to offspring, it needs not only to be inserted into the cell, but also to be incorporated into the chromosomes by genetic recombination.

• Somatic gene therapy can be broadly split in to two categories: ex vivo, which means exterior (where cells are modified outside the body and then transplanted back in again) and in vivo, which means interior (where genes are changed in cells still in the body). • Recombination-based approaches in vivo are especially uncommon, because for most DNA constructs recombination has a very low probability.

• Stem cells are the cells from which all other cells in your body are created. For gene therapy, stem cells can be altered in a lab to become cells that can help fight disease. • Liposomes. These fatty particles have the ability to carry the new, therapeutic genes to the target cells and pass the genes into your cells' DNA.

Embryonic stem cells to treat cancer: • Cancer has been one of the most debilitating diseases throughout the past hundred years. • Cancer is notoriously difficult to eradicate, mainly because it’s hard to detect in time and because there are so many different types of cancer out there. • Fortunately, new breakthroughs in stem cell research have seen the advent of embryonic stem cell lines that are capable of effectively treating some forms of cancer without the unwanted side effects linked to the most common available treatments. • While there is still a lot of work to do to perfect the treatments and develop additional stem cell lines, researchers are fairly confident that this line of study is one that could one day end cancer once and for all.

• Therapeutic cloning is another phrase for a procedure known as somatic cell nuclear transfer (SCNT). • An extraction of the nucleus from an egg • The nucleus holds the genetic material for a human or laboratory animal • The scientist then takes a somatic cell, which is any body cell other than an egg or sperm, and also extract the nucleus from this cell • In practical human applications, the somatic cell would be taken from a patient who requires a Stem Cell Transplant to treat a health condition or disease. • The nucleus that is extracted from the somatic cell in the patient is then inserted into the egg, which had its nucleus previously removed • In a very basic sense, it's a procedure of substitution. The egg now contains the patient's genetic material, or instructions • It is stimulated to divide and shortly thereafter forms a cluster of cells known as a blastocyst • This blastocyst has both an outer and inner layer of cells and it is the inner layer, called the inner cell mass that is rich in stem cells. The cells in the inner cell mass are isolated and then utilised to create embryonic stem cell lines, which are infused into the patient where they are ideally integrated into the tissues, imparting structure and function as needed.

Benefits of Therapeutic Cloning • A major benefit of therapeutic cloning is that the cells removed are pluripotent. • Pluripotent Cell can give rise to all cells in the body with the exception of the embryo. • This means that pluripotent cells can potentially treat diseases in any body organ or tissue by replacing damaged and dysfunctional cells. • Another distinct advantage to this type of therapy is that the risk of immunological rejection is alleviated because the patient's own genetic material is used. • If a cell line were created with cells from another individual, the patient's body would be more likely to recognise the foreign proteins and then wage an attack on the transplanted cells. • The ultimate consequence would be a rejected stem cell transplant. • This is one of the major challenges of organ transplants,

ADVANTAGES: 1. It has the potential to create organs. 2. Tissue rejection is no longer a threat. 3. It may help to treat genetic diseases. 4. Donor cells would no longer be necessary. 5. It could lead to organ regeneration. 6. It can act as a preventative treatment. 7. Therapeutic cloning could eliminate lengthy treatment times. 8. It offers new treatment options. Many long-term diseases could be immediately controlled, like diabetes. Devastating conditions, such as Alzheimer’s disease, could be treated.

DISADVANTAGES We must have a definitive definition of life. Life begins at conception. The idea of therapeutic cloning would therefore be tantamount to murder. There is always a threat of cell mutation. Mutations can occur spontaneously, even when using somatic cells for genetic transfer. These mutations have caused embryos to not divide as expected. Some have even created the threat of tumors. It would require an extensive supply of eggs. Egg extraction isn’t a comfortable procedure.
- Slides: 62