Medical Gases Manufacture Storage and Transport of Medical


























































- Slides: 90

Medical Gases: Manufacture, Storage, and Transport of Medical Gases Chapter 3 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

Outlines: I. Properties of Medical Gases: Air • Oxygen (O 2) • II. Storage and Transporting of Medical Gases • Carbon Dioxide (CO 2) • Cylinders Liquid Oxygen Systems • Helium ( He) • Medical Supply Systems • Nitric Oxide (NO) • Piping Systems • Nitrous Oxide (N 2 O) • Stations Outlet • O 2 Concentrators • 2 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

Learning Objectives Describe the chemical and physical properties of the medical gases most often encountered in respiratory care. Describe how medical gases and gas mixtures are produced. Discuss the clinical applications for medical gases and gas mixtures. Identify cylinder markings List the color codes used to identify medical gas cylinders. Calculate the duration of remaining contents of a compressed oxygen cylinder.

Learning Objectives (cont. ) Distinguish between gaseous and liquid storage methods. Calculate the duration of remaining contents of a liquid oxygen cylinder. Describe how to properly store, transport, and use compressed gas cylinders. Distinguish between gas supply systems. Describe what to do if a bulk oxygen supply system fails. Differentiate between safety systems that apply to various equipment connections. 4

Learning Objectives (cont. ) Select the appropriate devices to regulate gas pressure and/or control flow during various clinical settings. Describe how to assemble, check for proper function, and identify malfunctions in gas delivery equipment. Identify and correct common malfunctions of gas delivery equipment. 5

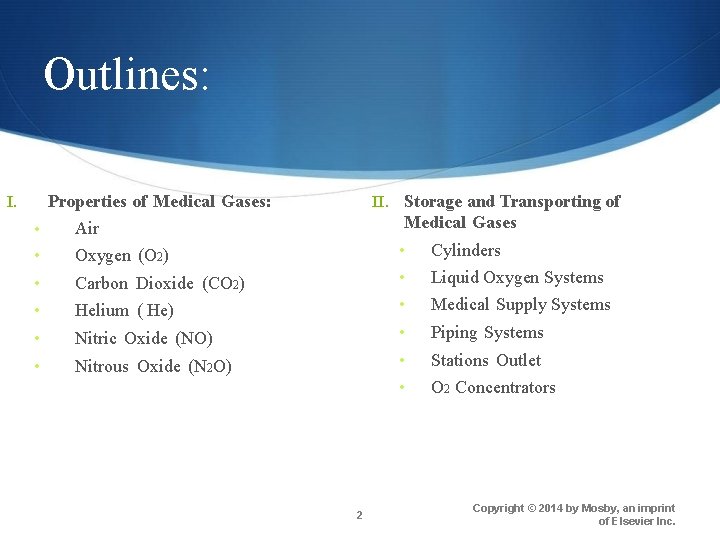
Air Colorless, odorless Contains water vapor Contains 20. 95% O 2, 78. 1% nitrogen, & ~1% trace gases (e. g. , Argon) Density 1. 2 kg/m 3 at 21. 1° C and 760 mm Hg Nonflammable, supports combustion Stored as gas in cylinders Medical-grade air produced by filtering & compressing atmospheric air 6 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

Air 7

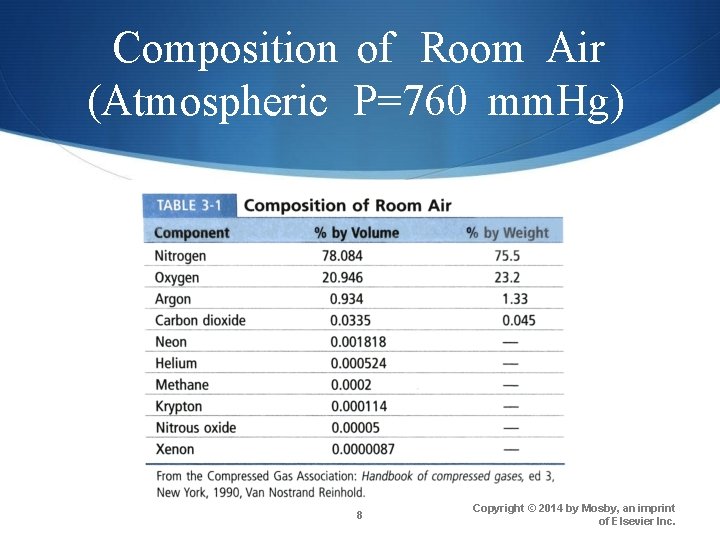
Composition of Room Air (Atmospheric P=760 mm. Hg) 8 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

Oxygen (O 2) Colorless, odorless, transparent, & tasteless Constitutes 50% of Earth’s crust Density 1. 326 kg/m 3 At temperatures< -183 C it becomes liquid Nonflammable but greatly accelerates combustion All elements , except inert, combine with O 2 to oxides Oxidizer Treats hypoxemia 9 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

Oxygen (O 2) O 2 Produced by: 1. Fractional distillation of liquid air 2. Physical separation of atmospheric air 10 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

Oxygen (O 2) Oxygen production 1. Fractional distillation Produces most large quantities of O 2 Atmospheric air is filtered to remove pollutants, water, & CO 2 Purified air is liquefied by compression & cooled by rapid expansion (Joule-Thompson effect) Resulting mixture is heated slowly to allow nitrogen to boil off, leaving just O 2

Oxygen (O 2) Oxygen production (cont. ) 2. Physical separation Molecular sieves absorb nitrogen, trace gases, & water vapor from air Oxygen concentrators pull ambient air through semipermeable plastic membrane 12

Carbon Dioxide (CO 2) Colorless, odorless gas Density 1. 833 kg/m 3 at 21. 1° C and 1 atm Nonflammable; does not support combustion Purified CO 2 prepared through: Liquefaction Fractional distillation Common uses for CO 2 mixtures Calibration of blood gas analyzers Diagnostic purposes in clinical laboratory 13 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

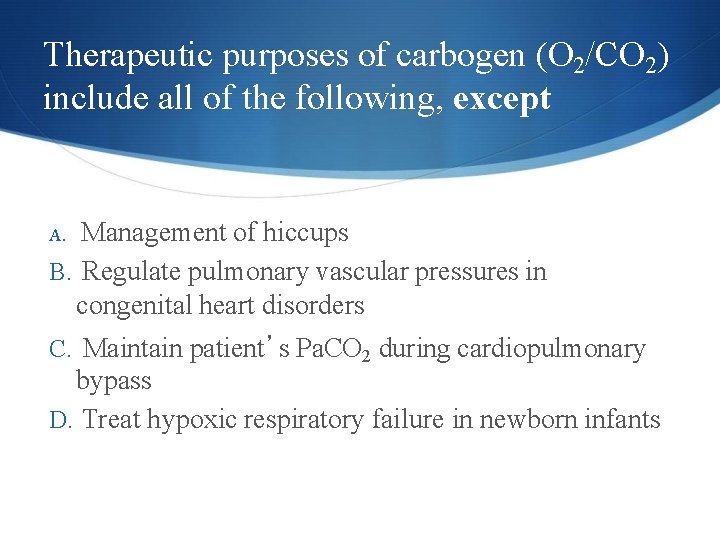
Carbon Dioxide (CO 2) Mixtures of O 2 & 5 -10% CO 2 have therapeutic purposes AKA: “carbogen” Management of singultus (hiccups) Prevent complete washout of CO 2 during cardiopulmonary bypass Regulate pulmonary vascular pressures in some congenital heart disorders 14 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

Therapeutic purposes of carbogen (O 2/CO 2) include all of the following, except Management of hiccups B. Regulate pulmonary vascular pressures in congenital heart disorders A. C. Maintain patient’s Pa. CO 2 during cardiopulmonary bypass D. Treat hypoxic respiratory failure in newborn infants 15

Helium (He) Inert, no color, odor, or taste Density 0. 165 kg/m 3 at 21. 1° C and 1 atm Nonflammable; non-life supporting Commercially produced from natural gas through liquefaction to purity standards of at least 99% Used in pulmonary function testing (PFT) for measuring residual volume (RV) and diffusing capacity 16 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

Helium (He) Therapeutic use Heliox (mixture of O 2 & helium) Manages severe cases of airway obstruction Decreases work of breathing Lower density Makes gas flow more laminar 17 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

Nitric Oxide (NO) Colorless gas, slight metallic odor Density: 1. 245 kg/m 3 Nonflammable; supports combustion Highly unstable in atmosphere Biologically active forms in tissues: NO+ (Nitrosonium), NO (Nitroxyl anions), and free radical NO Prepared by oxidizing ammonia at high temperatures Toxic at high concentrations (methemoglobinemia), but at low levels (2 to 80 parts per million) it acts as a pulmonary vasodilator Used to treat persistent pulmonary hypertension of the newborn (PPHN) and acute respiratory distress syndrome (ARDS) 18

A newborn infant with persistent pulmonary hypertension has been placed on mechanical ventilation. What special therapeutic gas would you recommend to improve the neonate’s condition? A. heliox B. nitrous oxide C. carbogen D. nitric oxide 19

Nitrous Oxide (N 2 O) Colorless, odorless, tasteless Nonflammable; supports combustion Oxidizing agent Prepared from: Thermal decomposition of ammonium nitrate Anesthetic (laughing gas) Risks Long-term exposure can lead to neuropathy issues Fetal disorders Spontaneous abortion 20

Storage and Transport of Medical Gases 21 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

Storage and Transport of Medical Gases Metal cylinders Liquid systems Medical air systems Central supply systems Station outlets Oxygen concentrators 22 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

Storage & Transport of Medical Gases Medical gases can be classified as liquefied and non- liquefied. Non-liquefied gases are stored and transported under high pressure in metal cylinders. Liquefied gases are stored and transported in bulk liquid storage Design, manufacture, transport, &use of these cylinders are controlled by industrial standards & federal regulations 23

Regulating Agencies CDRH – standards for medical devices DHHS – oversees health care delivery in U. S. DOT – regulations for manufacture, storage, and transportation of compressed gases EPA – standards/regulations of potential and actual environmental hazards FDA – sets purity standards for medical gases OSHA – oversees safety issues CGA – standards and safety systems for compressed-gas systems NFPA – provides information on fire protection and safety 24 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

Cylinder Construction Made of seamless steel, chrome-molybdenum, or aluminum Classified by U. S. Department of Transportation (DOT) Type 3 cylinders made from low-carbon steel, not used anymore. DOT type 3 A cylinders are made from carbon steel non heat treated DOT type 3 AA cylinders are made from steel alloy tempered for higher strength (heat treated) DOT type 3 AL cylinders are made from aluminum alloy 25 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

Cylinder Identification Engraved markings Color code Cylinder labels Why are all three used to identify a cylinder? Which one is the most reliable? 26 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

Markings and Identification Color coded & marked with metal stamping on shoulder Stamping indicates size, normal filling pressure, serial number, ownership, & method of manufacturer Safety tests are conducted every 5 or 10 years Cylinders are pressurized to five thirds of their service pressure Cylinder leakage, expansion, & wall stress are measured Results of pressure testing are stamped on tank 27

Engraved Markings 28

Color Codes for Medical Gases Copyright © 2014 by Mosby, an imprint of Elsevier Inc. 29

Gas Cylinders (cont. ) 30

Labels Primary way to identify cylinder contents 31

Cylinder Valves are control devices that seal the contents of a compressed cylinder until it is ready for use 32 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

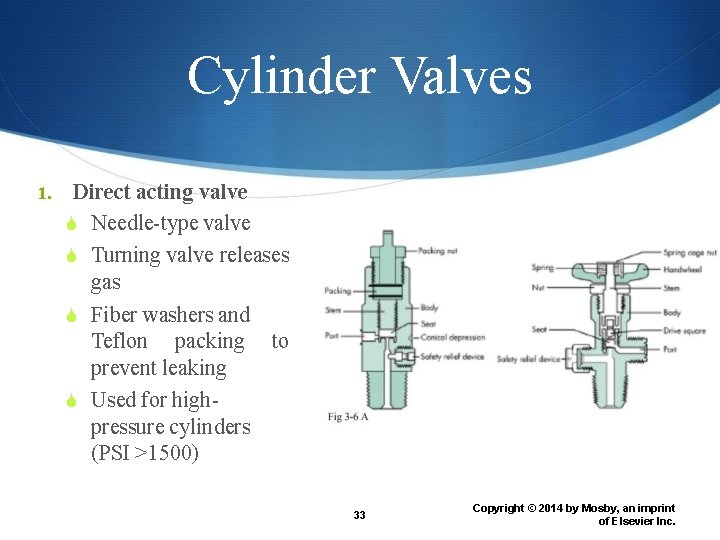
Cylinder Valves 1. Direct acting valve Needle-type valve Turning valve releases gas Fiber washers and Teflon packing to prevent leaking Used for highpressure cylinders (PSI >1500) 33 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

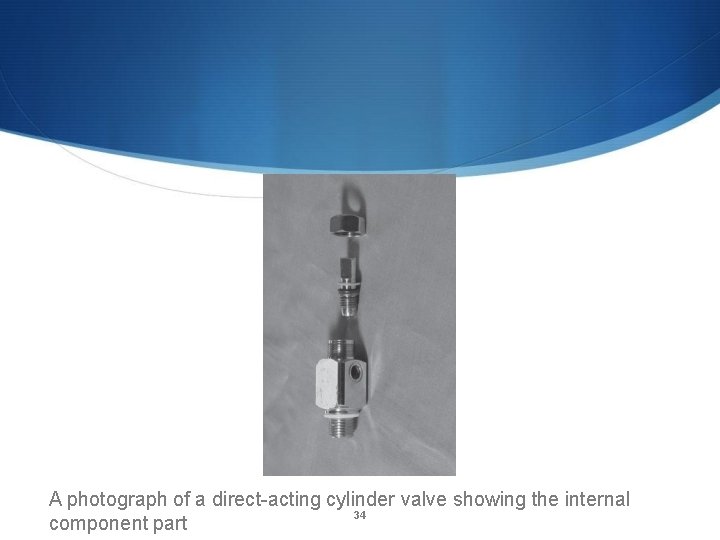
A photograph of a direct-acting cylinder valve showing the internal 34 component part

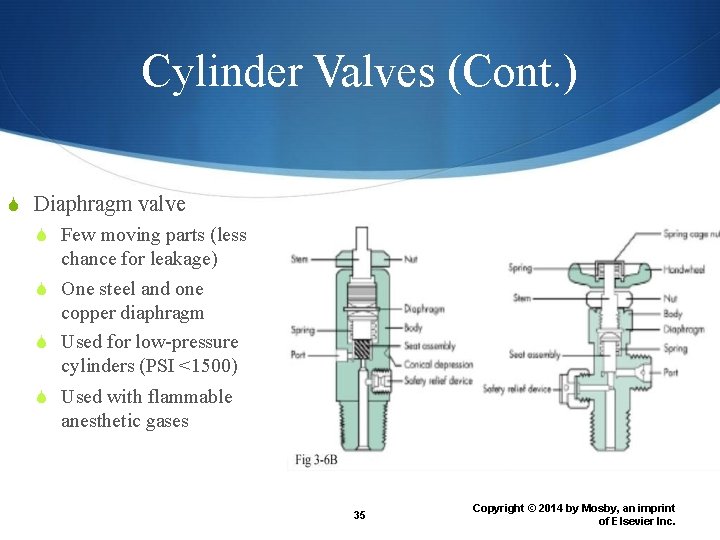
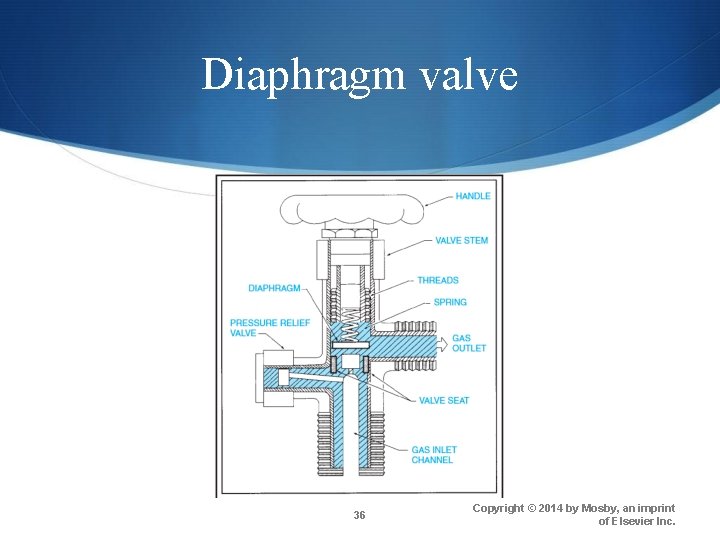
Cylinder Valves (Cont. ) Diaphragm valve Few moving parts (less chance for leakage) One steel and one copper diaphragm Used for low-pressure cylinders (PSI <1500) Used with flammable anesthetic gases 35 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

Diaphragm valve 36 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

Pressure Relief Valves Designed to vent gas to atmosphere if tank is heated Prevents tank pressure from becoming too high Basic designs Frangible metal disk ruptures at specific pressure Fusible plug melts at specific temperature Spring loaded valve opens & vents gas at set high pressure 37 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

38 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

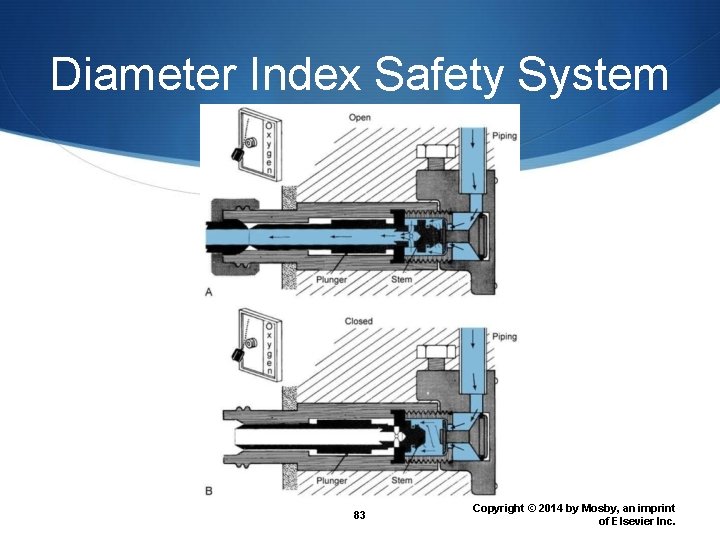
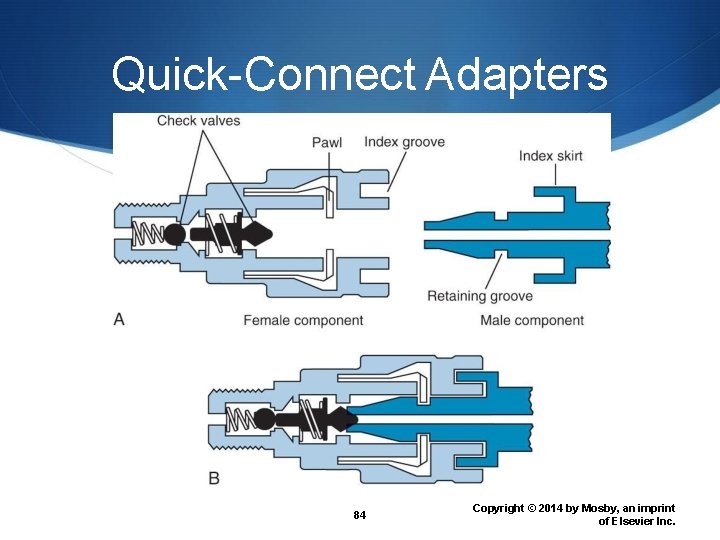
American Standard Indexing American Standard Index System (ASSS) Diameter indexing safety system (DISS) Pin indexing safety system (PISS) Quick-connect system Why are different safety systems available? 39 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

ASSS Used with large cylinders (size H and K) Used with gas connections where pressure >1500 psi Each gas has its own combination of: Right-handed or left-handed valves Internal or external connections No. of threads per inch Connector size, type, and seal configurations Medical gases = right-handed, external threads 40 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

PISS For high-pressure “A” through “E” size cylinders (>1500 psi) Does not use threads Uses a “yoke” with pins Each medical gas has its own PIN placement 2 - 5 for oxygen 1 - 5 for air 41 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

PISS for Small Cylinders 42

PISS 43

DISS For low-pressure gas connectors Found at: outlets of pressure-reducing valves outlets of central piping systems inlets of blenders, flowmeters, vents 44 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

DISS 45

46 Copyright © 2014 by Mosby, an imprint of Elsevi er Inc.

You are called to prepare an E-cylinder of oxygen for a patient. Which pinhole locations on a regulator should be used? A. B. C. D. 47 1 and 5 2 and 6 1 and 6

Procedure for Using Cylinders 1. Clean off cylinder 2. Ensure contents of cylinder 3. No grease or oil on gloves or hands 4. Remove protective cap 5. Point the valve outlet away from you and the patient 6. “Crack” the cylinder – open and close quickly before attaching to regulator 7. Replace the protective cap when not in use 48 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

Calculation of Gas Cylinder Content Full medical gas cylinders contain approximately 2200 psi Predicting length of time that cylinder will provide medical gas depends on: Volume of gas in cylinder Pressure exerted by compressed gas Flow rate of gas exiting cylinder 49 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

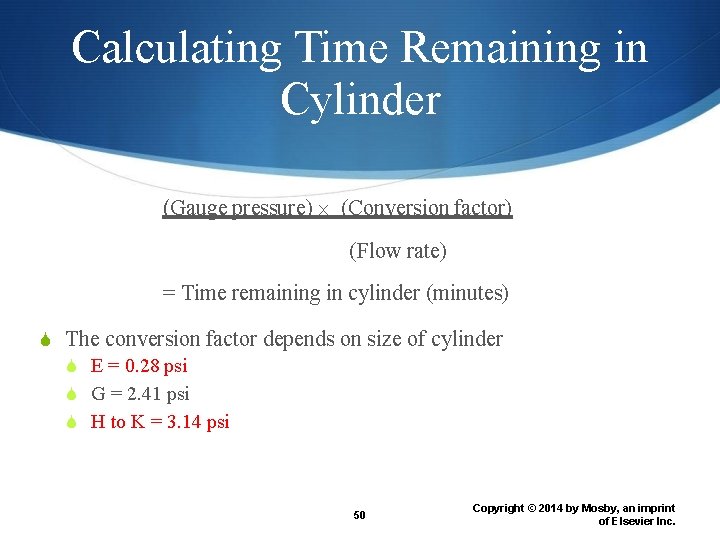
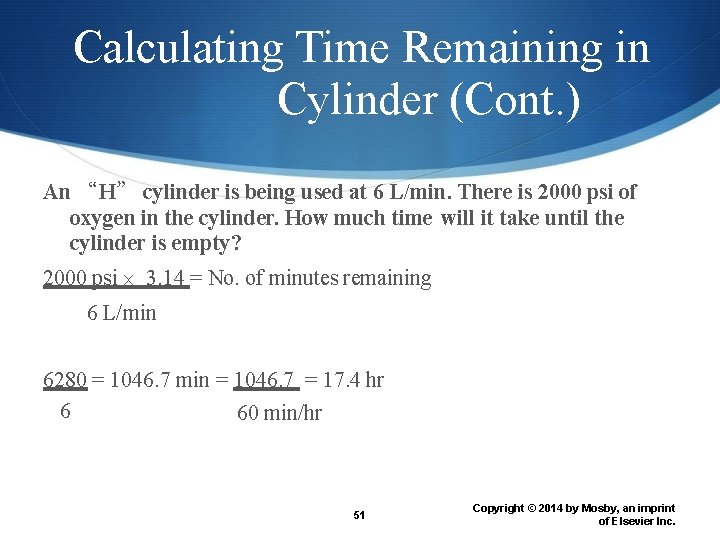
Calculating Time Remaining in Cylinder (Gauge pressure) (Conversion factor) (Flow rate) = Time remaining in cylinder (minutes) The conversion factor depends on size of cylinder E = 0. 28 psi G = 2. 41 psi H to K = 3. 14 psi 50 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

Calculating Time Remaining in Cylinder (Cont. ) An “H” cylinder is being used at 6 L/min. There is 2000 psi of oxygen in the cylinder. How much time will it take until the cylinder is empty? 2000 psi 3. 14 = No. of minutes remaining 6 L/min 6280 = 1046. 7 min = 1046. 7 = 17. 4 hr 6 60 min/hr 51 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

Examples 1. Calculate the time it will take to empty an “E” cylinder with 1100 psi, using 3 L/min. 2. Cylinders are replaced when they reach 500 psi. Calculate how long it would take for an “H” oxygen cylinder to reach that level if it is consuming 8 L/min, with 1850 psi. 52 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

Answer to Example 1 1100 x 0. 28 = 308 3 L/min or = 102. 7 min 3 102. 7 min = 1 hr 42. 6 min 60 min/hr 53 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

Answer to Example 2 No. of psi used until tank reaches 500 psi = 1850 − 500 = 1350 psi 1350 x 3. 14 = 4239 8 L/min 8 = 529. 9 min 60 min/hr = 8 hr 50 min 54 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

Why Do You Need To Calculate The Remaining Time? 55 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

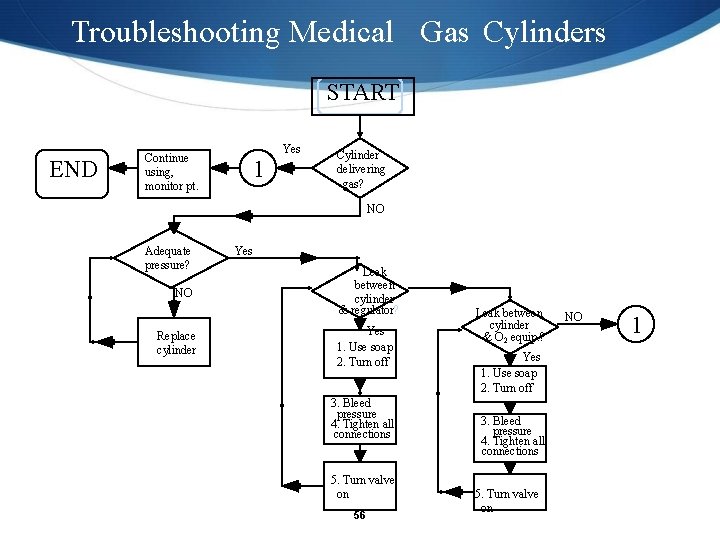
Troubleshooting Medical Gas Cylinders START END Continue using, monitor pt. 1 Yes Cylinder delivering gas? NO Adequate pressure? NO Replace cylinder Yes Leak between cylinder & regulator? Yes 1. Use soap 2. Turn off 3. Bleed pressure 4. Tighten all connections 5. Turn valve on 56 Leak between cylinder & O 2 equip. ? Yes 1. Use soap 2. Turn off 3. Bleed pressure 4. Tighten all connections 5. Turn valve on NO 1

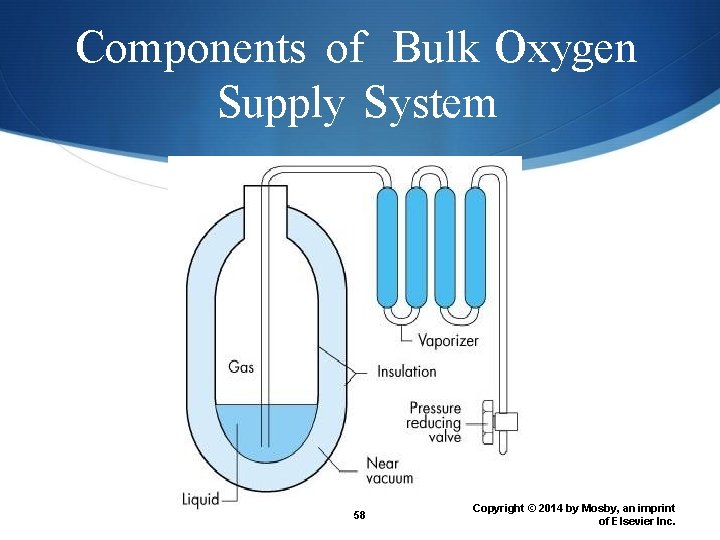
Liquid O 2 Systems Hospitals and larger health care facilities rely on bulk liquid supply systems for medical air and O 2. Temperature < 184° C ( 300° F) Gaseous O 2 is above liquid oxygen’s surface Gaseous O 2 occupies a volume of 860 times that of a liquid O 2 Liquid O 2 is stored in a thermos-like canister 57 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

Components of Bulk Oxygen Supply System 58 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

Portable Liquid O 2 System Smaller version of the bulk O 2 system are available for the home care setting. Stored in a thermos-like canister It has two components: a stationary base reservoir and a portable unit. The main unit contains a liquid reservoir, a vaporizer coil, and a pressure-relief valve. 59 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

Safety Guidelines for Liquid O 2 Systems Check line pressure (40 to 60 psi) and purity (99%) Be aware of construction projects Understand how the system and the backup system work Know hospital’s daily usage, perform daily reading of liquid O 2 levels (inches) 60 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

O 2 Supply Systems Bulk liquid O 2 system has more than 20, 000 ft 3 of O 2 ready for use Central supply O 2 system = two types Continuous Operating supply (liquid or gas) Reserve supply (liquid or gas) only in emergency Alternating Two alternating operating supplies 61 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

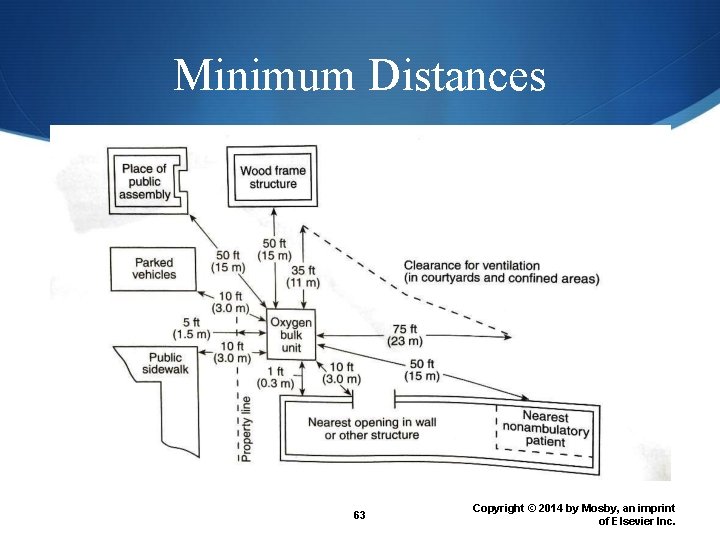
Bulk O 2 System Recommendations and Regulations National Fire Protection Association (NFPA) specifications include: 25 feet from a congested area 50 feet from a place of public assembly 25 feet from flammable gas storage 10 feet from the public sidewalk 62 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

Minimum Distances 63 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

Facts You Need to Know About Liquid Oxygen 1 L liquid O 2 weighs 2. 5 lb Liquid O 2 weight 2. 5 = liters of liquid O 2 gas occupies a volume 860 times the volume of liquid O 2 Liters of liquid O 2 × 860 = liters of O 2 gas 64 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

1 L of liquid oxygen Weighs 2. 5 lb 65 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

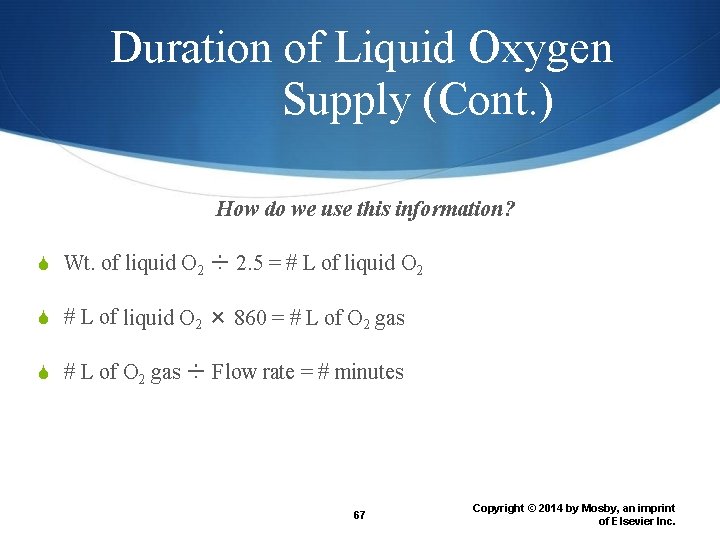
Duration of Liquid Oxygen Supply What do we know about a portable system? Weight of the empty container Current weight of container Flow rate of gaseous O 2 exiting the system 66 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

Duration of Liquid Oxygen Supply (Cont. ) How do we use this information? Wt. of liquid O 2 ÷ 2. 5 = # L of liquid O 2 × 860 = # L of O 2 gas ÷ Flow rate = # minutes 67 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

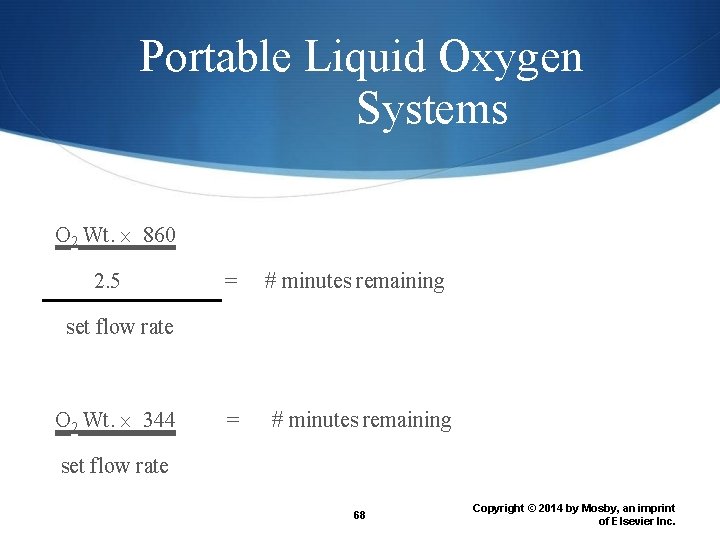
Portable Liquid Oxygen Systems O 2 Wt. 860 2. 5 = # minutes remaining set flow rate O 2 Wt. 344 = # minutes remaining set flow rate 68 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

Duration Example • Empty weight = 10 lb • Current weight = 25 lb • Usage = 3 L/min • How many days are left? 69 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

Answer 25 − 10 = 15 lb of liquid O 2 (15 × 344) ÷ 3 L/min = 1720 min or 28. 7 hr or 1. 2 days 70 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

Medical Air Supply Portable air compressors Bulk air supply systems 71 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

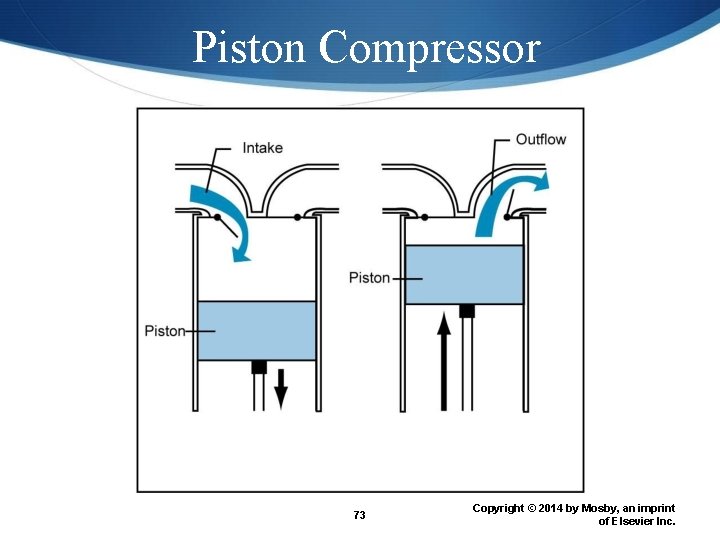
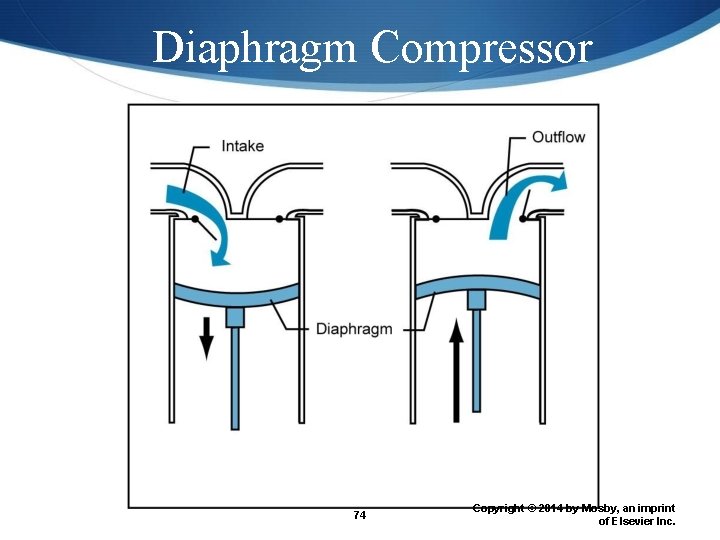
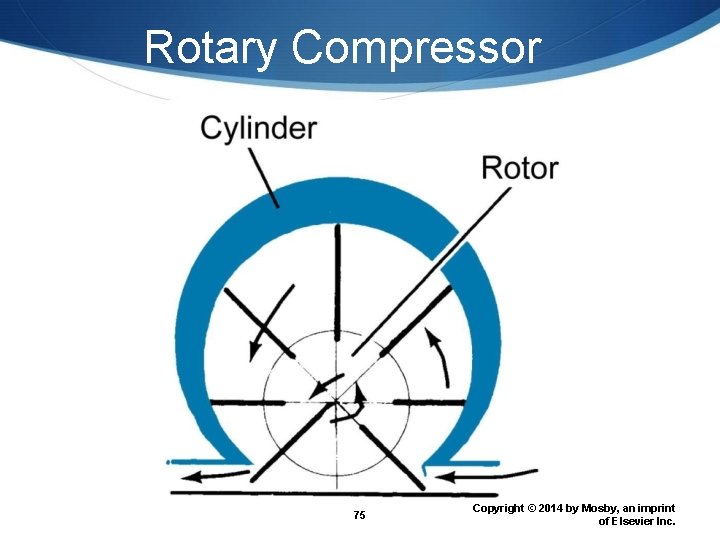
Portable Air Compressors Piston compressors Diaphragm compressors Rotary compressors 72 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

Piston Compressor 73 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

Diaphragm Compressor 74 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

Rotary Compressor 75 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

Bulk Air Supply Systems Hospitals and other health care facilities Two compressors Operate together Operate independently Each able to deliver 100% of average peak demand Usually piston or rotary Working pressure 50 psi 76 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

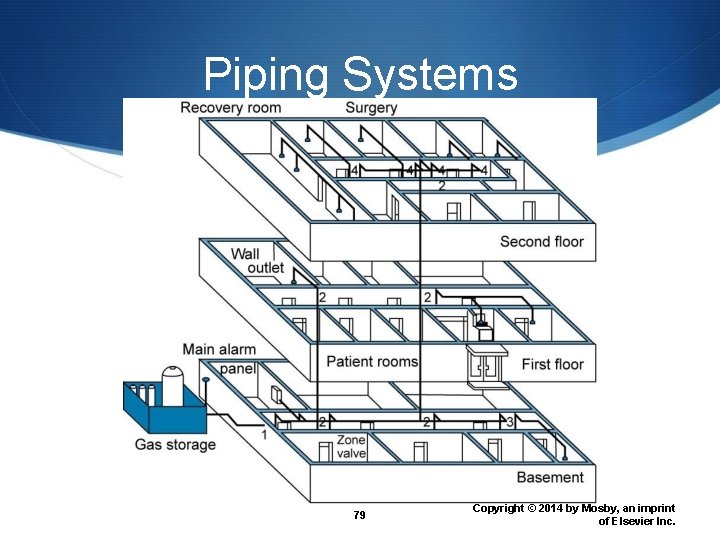
Central Supply System Provide medical gases to multiple sites within an institution Continuous supply system Alternating supply system Piping system 77 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

Continuous Supply System Two sources of gas supply One primary source Large source usually liquid Refilled regularly One reserve for emergency Smaller liquid reservoir or bank of compressed gas cylinders At least 24 -hour reserve 78 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

Piping Systems 79 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

Zone Valve 80 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

Station Outlets Provide connections for gas-delivery devices Flowmeters Mechanical ventilators Parts Body mounted to supply line Outlet faceplate Primary and secondary check valves (open when device connected and close when removed) Color coded 81 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

Station Outlets (Cont. ) Safety systems Diameter index safety system (DISS) Quick-connect adapters 82 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

Diameter Index Safety System 83 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

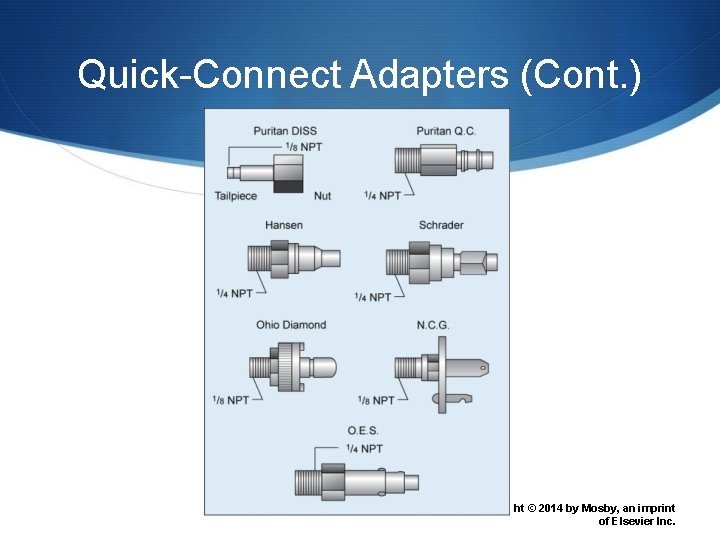
Quick-Connect Adapters 84 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

Quick-Connect Adapters (Cont. ) 85 Copyrig ht © 2014 by Mosby, an imprint of Elsevier Inc.

Station Outlets 86 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

Oxygen Concentrators Alternative to compressed-gas systems in the home Two types Semipermeable plastic membranes Molecular sieve 87 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

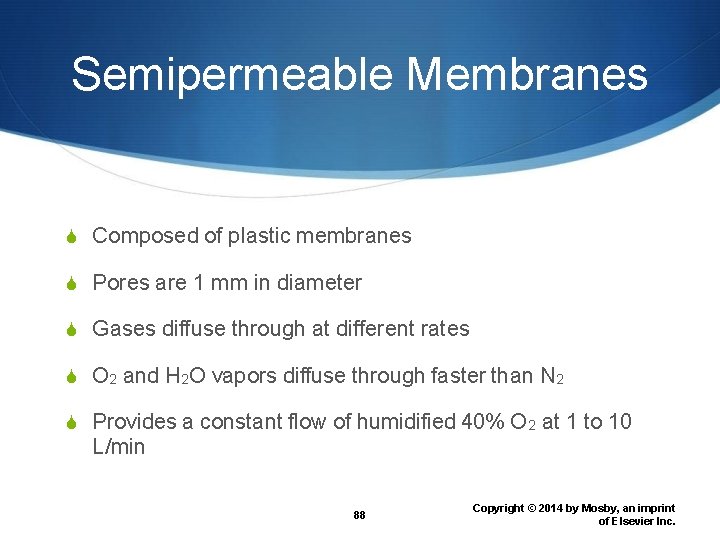
Semipermeable Membranes Composed of plastic membranes Pores are 1 mm in diameter Gases diffuse through at different rates O 2 and H 2 O vapors diffuse through faster than N 2 Provides a constant flow of humidified 40% O 2 at 1 to 10 L/min 88 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

Molecular Sieve Sodium-aluminum silicate (zeolite) pellets Remove N 2 and other gases Must be purged to remove N 2 and other gases FIO 2 depends on flow rate set <6 L/min provides 92% to 97% O 2 89 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.

Summary Respiratory therapists must be familiar with medical gas Properties both gaseous and liquid Manufacture of gases Storage systems Transport Safety systems Delivery in health care systems 90 Copyright © 2014 by Mosby, an imprint of Elsevier Inc.