Medical Device Risk Management Practical Overview Challenges Aruna






- Slides: 10

Medical Device Risk Management: Practical Overview & Challenges Aruna Ranaweera, Ph. D Corporate Process Owner – Design Controls & Risk Management Stryker Corporation October 2, 2012 – San Francisco, CA

Focus on Patients Manufacturer’s viewpoint The intended use/purpose of a medical device can be depicted using an idealized functional input/output diagram: “Engineering World” Functional Inputs Functional Outputs Medical Device User (Operator) “Clinical World” Medical Benefit Time Patient

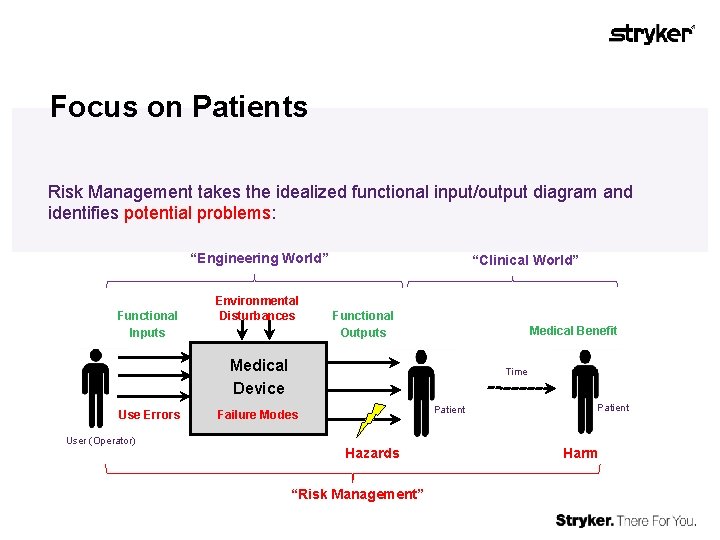
Focus on Patients Risk Management takes the idealized functional input/output diagram and identifies potential problems: “Engineering World” Functional Inputs Environmental Disturbances “Clinical World” Functional Outputs Medical Benefit Medical Device Use Errors User (Operator) Time Patient Failure Modes Hazards “Risk Management” Patient Harm

International Standard for Medical Device Risk Management ISO 14971, 2 nd edition: Medical Devices – Application of Risk Management to Medical Devices (2007) ISO 14971 is required by … USA - Food and Drug Administration European Union - Medical Device Directive 93/42/EEC

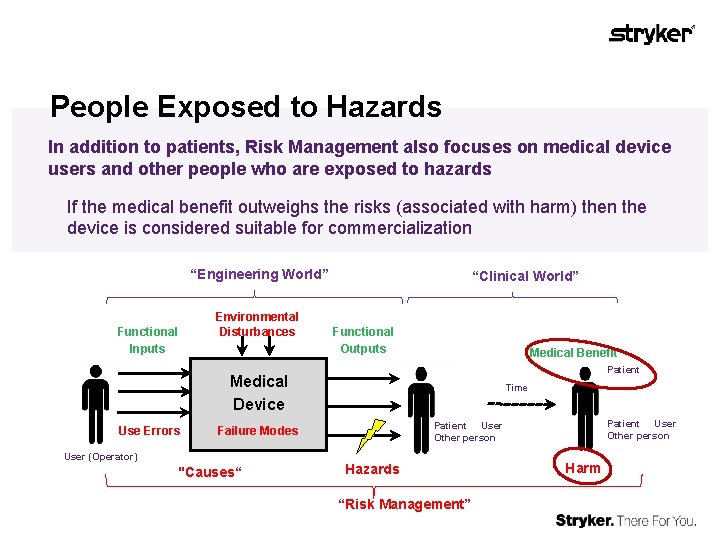
People Exposed to Hazards In addition to patients, Risk Management also focuses on medical device users and other people who are exposed to hazards If the medical benefit outweighs the risks (associated with harm) then the device is considered suitable for commercialization “Engineering World” Environmental Disturbances Functional Inputs “Clinical World” Functional Outputs Medical Benefit Patient Medical Device Use Errors Time User (Operator) "Causes“ Patient User Other person Failure Modes Hazards “Risk Management” Harm

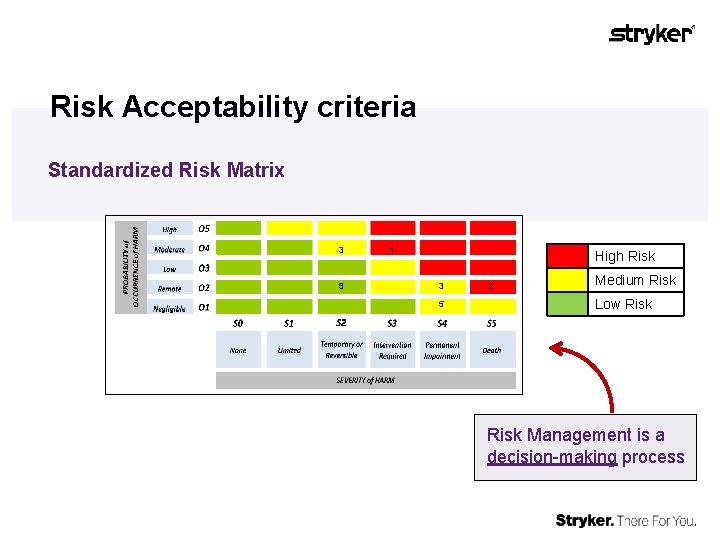
Risk Acceptability criteria Standardized Risk Matrix 3 9 1 High Risk 3 5 2 Medium Risk Low Risk Management is a decision-making process

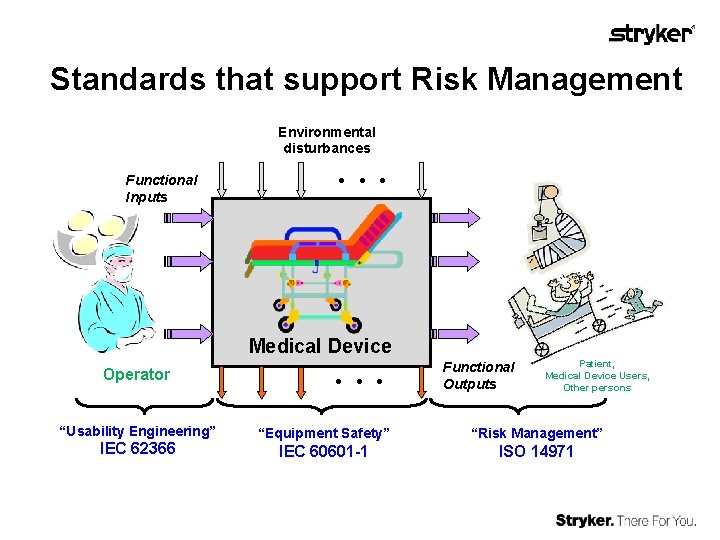
Standards that support Risk Management Environmental disturbances Functional Inputs Medical Device Operator “Usability Engineering” IEC 62366 Functional Outputs Patient, Medical Device Users, Other persons “Equipment Safety” “Risk Management” IEC 60601 -1 ISO 14971

IEC 60601 -1, 3 rd edition (2005) Ensures that devices meets minimum safety requirements, but does not address all risks Requires a robust Risk Management process per ISO 14971 IEC 60601 -1 Industry Challenges: 1. Standard is long and unwieldy 2. Some requirements are difficult to fully understand 3. Standard was recently amended (2012 July) 1.

Risk Management Challenge Complicated medical systems are: 1. Difficult to fully analyze 2. Not fully covered by safety standards

Summary Risk Mgmt standard ISO 14971 ensures that medical device risks are acceptable • ISO 14971 is relatively straightforward and practical • Risk management can be difficult for complicated systems “Equipment safety” standard IEC 60601 -1 ensures that minimum equipment safety requirements are met • IEC 60601 -1 is difficult to fully understand 1.