Medical Device Regulatory Reimbursement and Compliance Congress Steve

Medical Device Regulatory, Reimbursement and Compliance Congress Steve Ubl President and CEO Adva. Med March 28, 2007

Overview of Remarks • About Adva. Med • The Public Policy Environment • Adva. Med’s Priorities for 2007

About Adva. Med • World’s largest medical technology association • 1, 300+ member companies and subsidiaries • Members produce 90% of sales in domestic market, 50% of sales in global market • 70%+ of member companies have less than $30 million in annual revenue • 70 staff with global expertise, bi-partisan backgrounds • 45 member Board of Directors (BD, Siemens, Philips, Medtronic, Johnson & Johnson, GE, Boston Scientific, Roche, etc. )
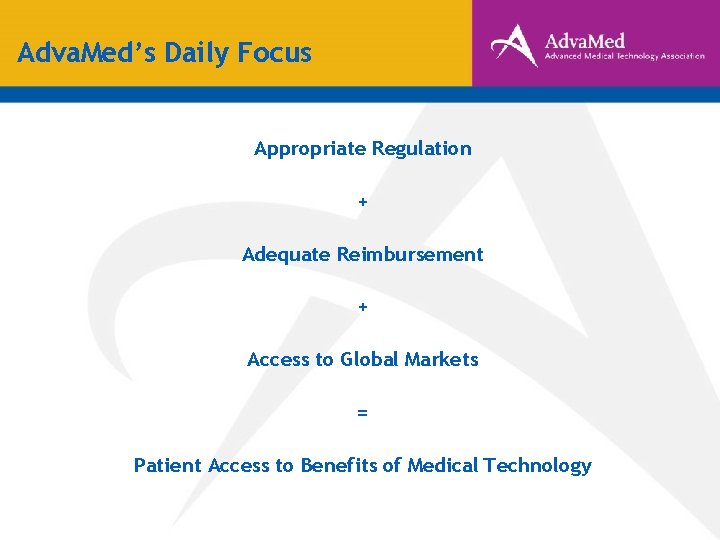
Adva. Med’s Daily Focus Appropriate Regulation + Adequate Reimbursement + Access to Global Markets = Patient Access to Benefits of Medical Technology
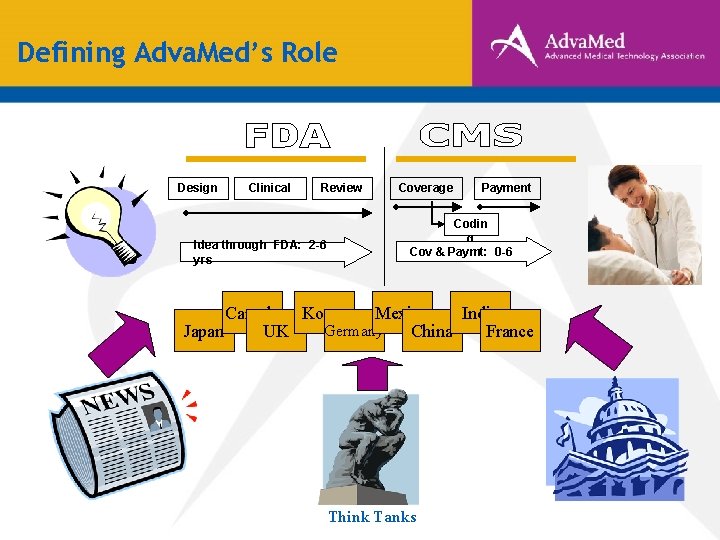
Defining Adva. Med’s Role Design Clinical Review Idea through FDA: 2 -6 yrs Coverage Payment Codin g Cov & Paymt: 0 -6 Canada Korea Mexico India Germany Japan UK China France Think Tanks

Policy Environment

Policy Environment

Policy Environment, cont’d The Legislative Front • More industry oversight • Critically important legislation pending ― Federal budget proposal with deep Medicare cuts ― SCHIP ― Physician Fee Fix ― Consideration of MDUFMA, PDUFA, drug safety, others will mean major FDA bill

Priorities for 2007
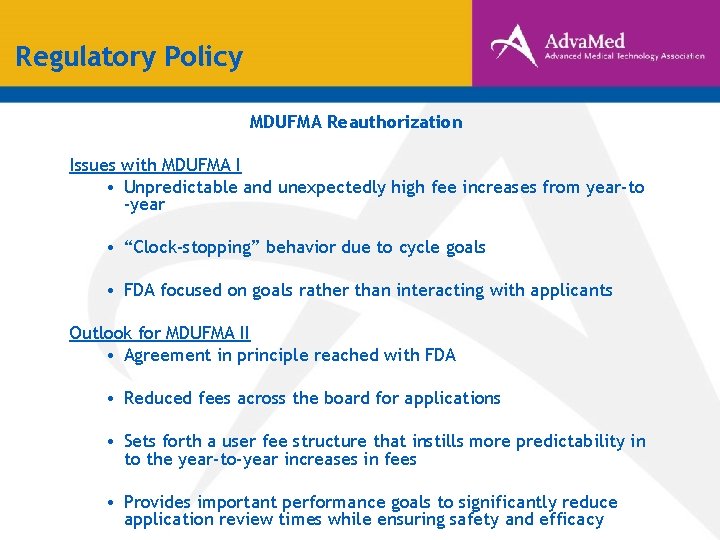
Regulatory Policy MDUFMA Reauthorization Issues with MDUFMA I • Unpredictable and unexpectedly high fee increases from year-to -year • “Clock-stopping” behavior due to cycle goals • FDA focused on goals rather than interacting with applicants Outlook for MDUFMA II • Agreement in principle reached with FDA • Reduced fees across the board for applications • Sets forth a user fee structure that instills more predictability in to the year-to-year increases in fees • Provides important performance goals to significantly reduce application review times while ensuring safety and efficacy

Regulatory Policy, continued • Post Market Regulation – Inappropriate comparison between drugs and devices – Adva. Med working to • Assist FDA in streamlining and “connecting the dots” with adverse event reports • Establish clearer criteria for communicating risk – Limit use of “recall” to appropriate situations • Develop flexible approach to Unique Device Identifiers (UDI)

Key Payment Rules • Inpatient Round II ― Severity weighted diagnosis related groups (DRGs) ― Hospital-specific relative values (HSRVs) ― Charge compression adjustment possible • Competitive Bidding ― Potential for cheapest is best approach to technology ― Inappropriately group different technologies together for bidding purposes ― Could deny patients access to best care

Value-based Purchasing • Legislative initiatives likely – demonstrations ongoing ― advocated by key health policy makers / analysts • If done correctly ― Improves quality ― Enhances diffusion of innovative technologies ― Rewards quality and efficiency • If done incorrectly ― “Efficiency” can become code for “cheapest is best” ― Freezes technology in place Value = Price + Quality

Key Legislation • Remote Monitoring ― Prime example of payment policy not keeping pace with technology ― Offers physicians real-time, remotely accessed patient information ― Provides homebound and rural patients 24/7 link to health care ― Legislation would eliminate disincentives in current Medicare rules that only provide payment for face-to-face meetings between patients and their doctors. Courtesy: Philips Patient using an ECG/Rhythm strip recorder, a blood pressure cuff and a Tele. Station that sends data via modem from the telestation to a server, then on to the care manager.
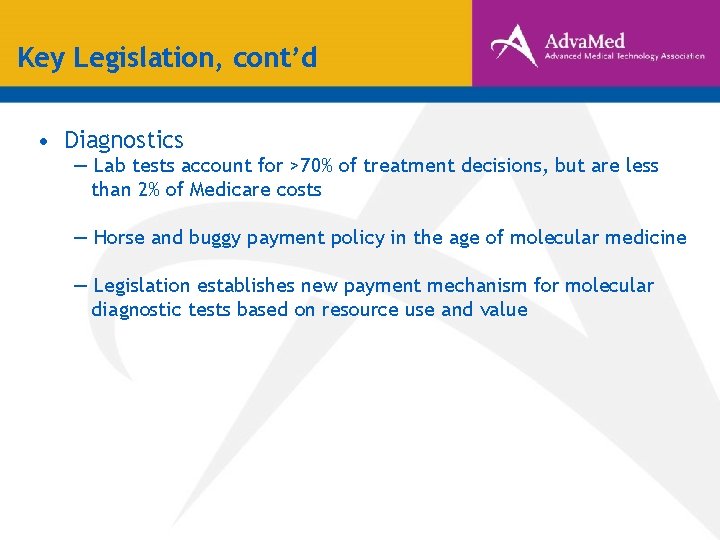
Key Legislation, cont’d • Diagnostics ― Lab tests account for >70% of treatment decisions, but are less than 2% of Medicare costs ― Horse and buggy payment policy in the age of molecular medicine ― Legislation establishes new payment mechanism for molecular diagnostic tests based on resource use and value

Health Reform Principles Shaping the future instead of being shaped by it. • Expand health coverage to all, so that every American have access to the best medicine has to offer • Improve the efficiency and quality of health care – Quality of care highest priority – Control cost the right way: lift the burden of disease and improve efficiency • Prevention • Quality • Efficiency • Medical innovation

International Top Line • Nascent health care economies: China, India • Japan: Foreign reference pricing • EU: DRGs and procurement policies – Europeans have followed US model for hospital payment based DRGs (France, Germany, UK, Italy)
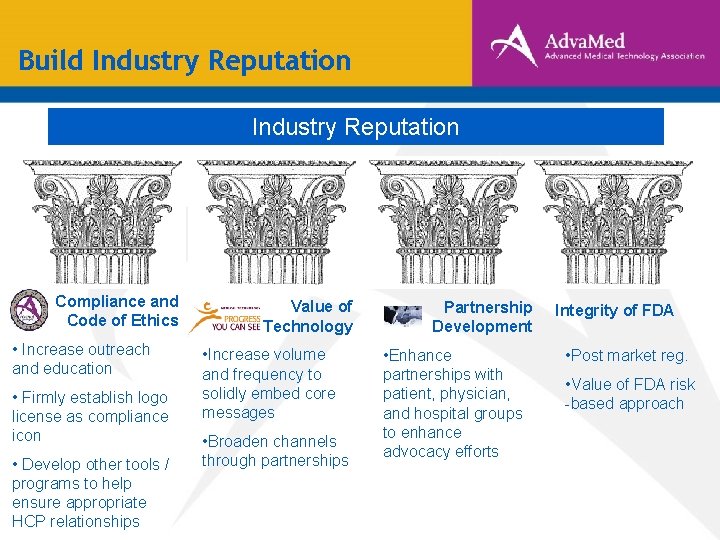
Build Industry Reputation Compliance and Code of Ethics • Increase outreach and education • Firmly establish logo license as compliance icon • Develop other tools / programs to help ensure appropriate HCP relationships Value of Technology • Increase volume and frequency to solidly embed core messages • Broaden channels through partnerships Partnership Development • Enhance partnerships with patient, physician, and hospital groups to enhance advocacy efforts Integrity of FDA • Post market reg. • Value of FDA risk -based approach

Telling our story

Medical Device Regulatory, Reimbursement and Compliance Congress Steve Ubl President and CEO Adva. Med March 28, 2007
- Slides: 20