Medical Device Innovation Early feasibility studies and beyond

Medical Device Innovation: Early feasibility studies and beyond Pieter Kappetein, CMO, VP Medical Affairs Medtronic

I, Pieter Kappetein, MD, Ph. D. I have relevant financial relationships: Full time employee of Medtronic

Introduction FDA’s mission is not only to protect the public health…. but also to advance the public health

Measure of success of Center for Devices and Radiological Health Responsible for premarket approval of medical devices, and overseeing manufacturing, performance and safety By 2021 more than 50% of manufacturers of novel technologies bring their devices to the U. S. first or in parallel with other major markets 3
Foster environment of Work closely with clinical community, patients and industry to allow speedy access to innovative devices Apply least burdensome approach“The minimum amount of information necessary to adequately address a regulatory question or issue through the most efficient manner at the right time” (e. g. , need to know versus nice to know) Medical device innovation starts with clinical research 4

FDA Clinical Trials Program Early Feasibility Study Program Encourages interaction Reduced review periods Consistent decision making

FDA Early Feasibility Study Program 2015 -2017 >50 Company Participants >120 Early Feasibility IDEs ~50% Increase in Annual # of EFS IDEs
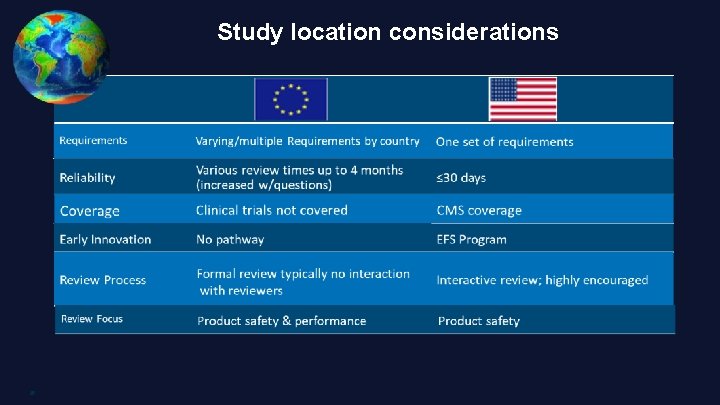
Study location considerations 8
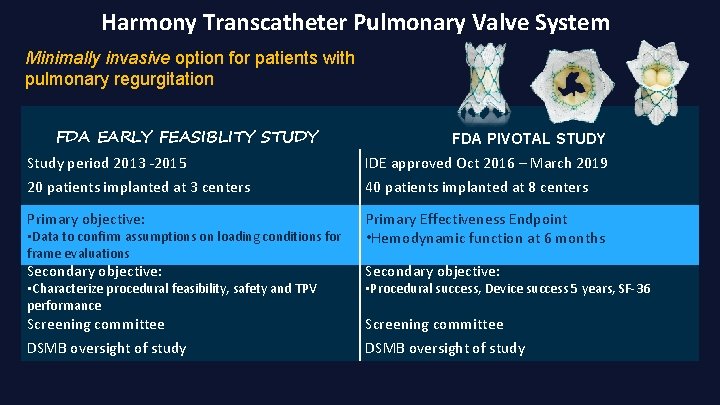
Harmony Transcatheter Pulmonary Valve System Minimally invasive option for patients with pulmonary regurgitation FDA EARLY FEASIBLITY STUDY FDA PIVOTAL STUDY Study period 2013 -2015 IDE approved Oct 2016 – March 2019 20 patients implanted at 3 centers 40 patients implanted at 8 centers Primary objective: Primary Effectiveness Endpoint • • Hemodynamic function at at 66 months Secondary objective: Screening committee DSMB oversight of study • • Data to to confirm assumptions on on loading conditions for frame evaluations • Characterize procedural feasibility, safety and TPV performance • Procedural success, Device success 5 years, SF-36
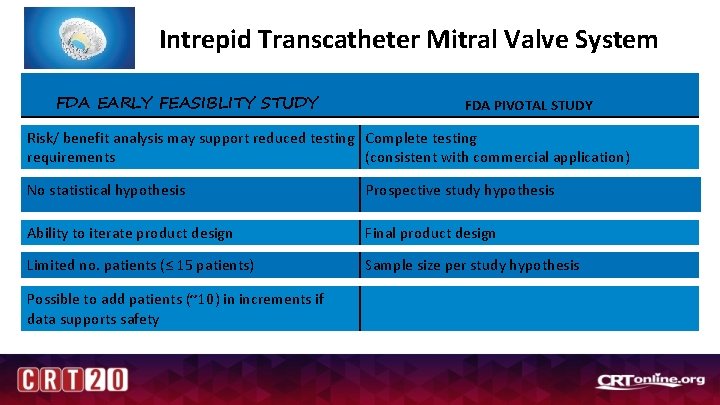
Intrepid Transcatheter Mitral Valve System FDA EARLY FEASIBLITY STUDY FDA PIVOTAL STUDY Risk/ benefit analysis may support reduced testing Complete testing requirements (consistent with commercial application) No statistical hypothesis Prospective study hypothesis Ability to iterate product design Final product design Limited no. patients (≤ 15 patients) Sample size per study hypothesis Possible to add patients (~10) in increments if data supports safety
Early Feasibility Study EXPERIENCE 1 Proof of concept with ability to iterate quickly 2 Informs product and pivotal study design 3 FDA familiarity with product, design changes and data 4 Data filed with EFS that continues to support final design can be referenced 5 Facilitates smooth transition from EFS to Pivotal 6 Develops product competence at EFS sites 7 CMS coverage can be obtained in parallel with EFS

CHALLENGES • FDA EFS to Pivotal process delivers on promises • Timeline to site approvals remains the gating item with site contracts taking up to 9 months • For larger companies, Pivotal studies are the key to global market expansion • Acceleration of EFS to Pivotal can exclude regions beyond the US • Companies quality system is not necessarily designed to accommodate EFS concept

One • FDA transition from EFS to Pivotal is a smooth process Conclusions Two • Broader eco-system can be further developed to facilitate overall EFS model Three • Expansion to include global study paradigm is a key consideration


FDA Clinical Trials Program • • Consistent decision making Reduced review periods Encourages interaction Early Feasibility Study Program ¡ Earliest patient access ¡ Close collaboration between developers & users ¡ Clinical study continuity from early clinical use to pivotal to postapproval
- Slides: 15